Your Potassium symbol of ion images are available. Potassium symbol of ion are a topic that is being searched for and liked by netizens today. You can Find and Download the Potassium symbol of ion files here. Find and Download all free images.
If you’re searching for potassium symbol of ion images information connected with to the potassium symbol of ion topic, you have pay a visit to the ideal blog. Our site frequently gives you suggestions for downloading the maximum quality video and image content, please kindly search and locate more enlightening video articles and graphics that match your interests.
Potassium Symbol Of Ion. Give the symbol of an ion that has 10 e - and 7 p. The symbol and charge of potassium ion is K. Potassium ion K CID 813 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists. Classified as a n alkali metal Potassium is a solid at room temperature.
![]() Potassium Ion Images Stock Photos Vectors Shutterstock From shutterstock.com
Potassium Ion Images Stock Photos Vectors Shutterstock From shutterstock.com
It is formed when the element chlorine gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. K potassium kalium K potassium ion Rb rubidium Rb rubidium ion Cs cesium Cs cesium ion Be beryllium Be 2 beryllium ion Mg. This element is nitrogen which has the symbol N. The chemistry of potassium is almost etirely that of the potassium ion K. In the periodic table potassium is one of the alkali metals. We have to interchange the number of positive charges and the number of negative charges.
K potassium kalium K potassium ion Rb rubidium Rb rubidium ion Cs cesium Cs cesium ion Be beryllium Be 2 beryllium ion Mg.
There is an ion channel that allows for the. Potassium ion K CID 813 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists. Potassium has to lose 1 electron in order to form an ion. A potassium atom loses one electron. Physiologically it exists as an ion in the body. Using this the chemical formula of potassium oxide can be found as Potassium.
 Source: slideplayer.com
Source: slideplayer.com
Potassium is a chemical element with atomic number 19 which means there are 19 protons and 19 electrons in the atomic structure. K potassium kalium K potassium ion Rb rubidium Rb rubidium ion Cs cesium Cs cesium ion Be beryllium Be 2 beryllium ion Mg. From the Periodic T. Chloride salts such as sodium chloride are often very soluble in water. In this video well use the Periodic table and a few simple rules to find the number of protons and electrons for the Potassium ion K.
![]() Source: shutterstock.com
Source: shutterstock.com
The chloride ion ˈklɔːraɪd is the anion Cl. Is Phosphate an anion or cation. This work is done by the NaK ATPase pump which pumps 3 Na ions out of the cell and 2K into the cell to generate the Na and K concentration gradient. The chemistry of potassium is almost etirely that of the potassium ion K. In this video well use the Periodic table and a few simple rules to find the number of protons and electrons for the Potassium ion K.
 Source: slidetodoc.com
Source: slidetodoc.com
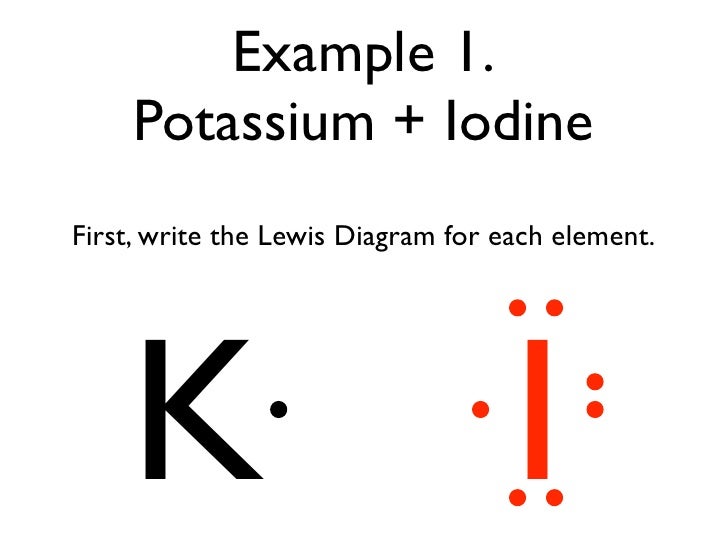
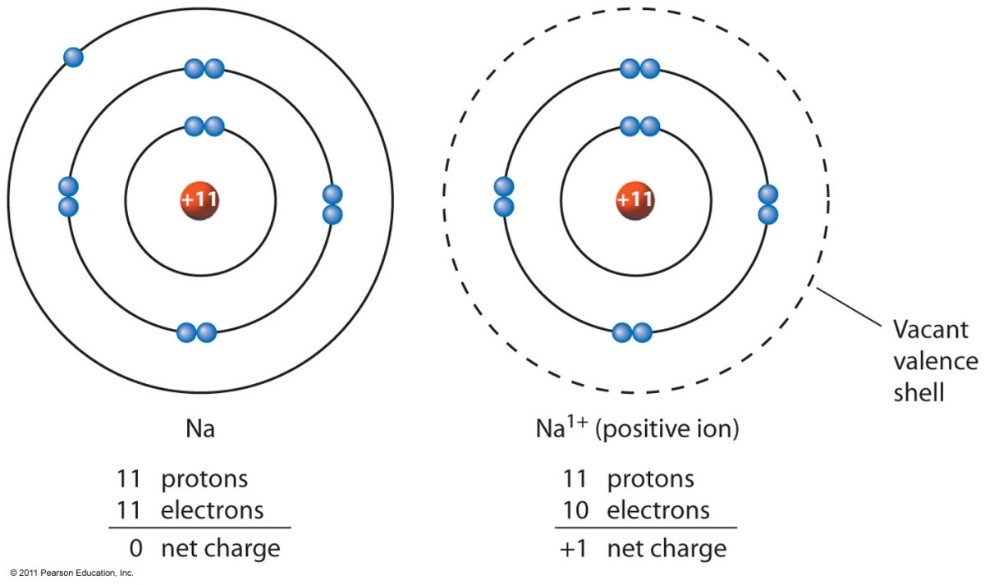
Since it only needs to get rid of one electron the ion will be a cation with a charge of 1 which means the symbol will be K. Potassium was first isolated from potash the ashes of plants from which its name derives. In the periodic table potassium is one of the alkali metals. Study About Potassium Chromate Formula. Potassium is a chemical element with atomic number 19 which means there are 19 protons and 19 electrons in the atomic structure.
 Source: docbrown.info
Source: docbrown.info
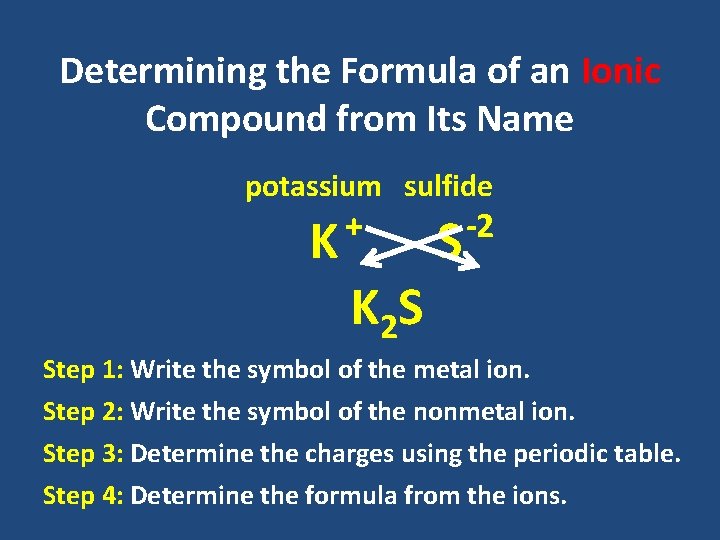
A potassium atom loses one electron. A flourine atom gains one electron. What is the electron configuration of a potassium ion. A potassium atom loses one electron. Most potassium 95 goes into fertilizers and the rest goes mainly into making potassium hydroxide KOH by the electrolysis of potassium chloride solution and then converting this to potassium carbonate K 2 CO 3.
 Source: flinnsci.com
Source: flinnsci.com
We have to interchange the number of positive charges and the number of negative charges. The chloride ion ˈklɔːraɪd is the anion Cl. A zinc atom loses two electrons. The number of protons is an elements atomic number. Chloride salts such as sodium chloride are often very soluble in water.
 Source: slideshare.net
Source: slideshare.net
A zinc atom loses two electrons c. There is an ion channel that allows for the. Hence it is due a small salty herb that we now end up with the symbol K for the element pot-ash-ium. A fluorine atom gains one electron Write the charge and symbol in the chart below when an atom forms an ion. Classified as a n alkali metal Potassium is a solid at room temperature.
 Source: alevelbiology.co.uk
Source: alevelbiology.co.uk
Give the symbol of an ion that has 10 e - and 7 p. A potassium atom loses one electron b. The chloride ion ˈklɔːraɪd is the anion Cl. Why does potassium have 19 electrons. The second is that the membrane is semipermeable to that ion.
 Source: steemit.com
Source: steemit.com
This element is nitrogen which has the symbol N. This salt can appear as colourless crystals crystalline powder in white or white grains under standard temperature and pressure. Why does potassium have 19 electrons. We have to interchange the number of positive charges and the number of negative charges. But in order for this process to occur a concentration gradient of potassium ions must first be set up.
 Source: slideplayer.com
Source: slideplayer.com
The symbol and charge of potassium ion is K. Physiologically it exists as an ion in the body. What is the electron configuration of a potassium ion. A fluorine atom gains one electron Write the charge and symbol in the chart below when an atom forms an ion. So if you look for potassium symbol K in the periodic table you will find that it has the atomic number of 19.
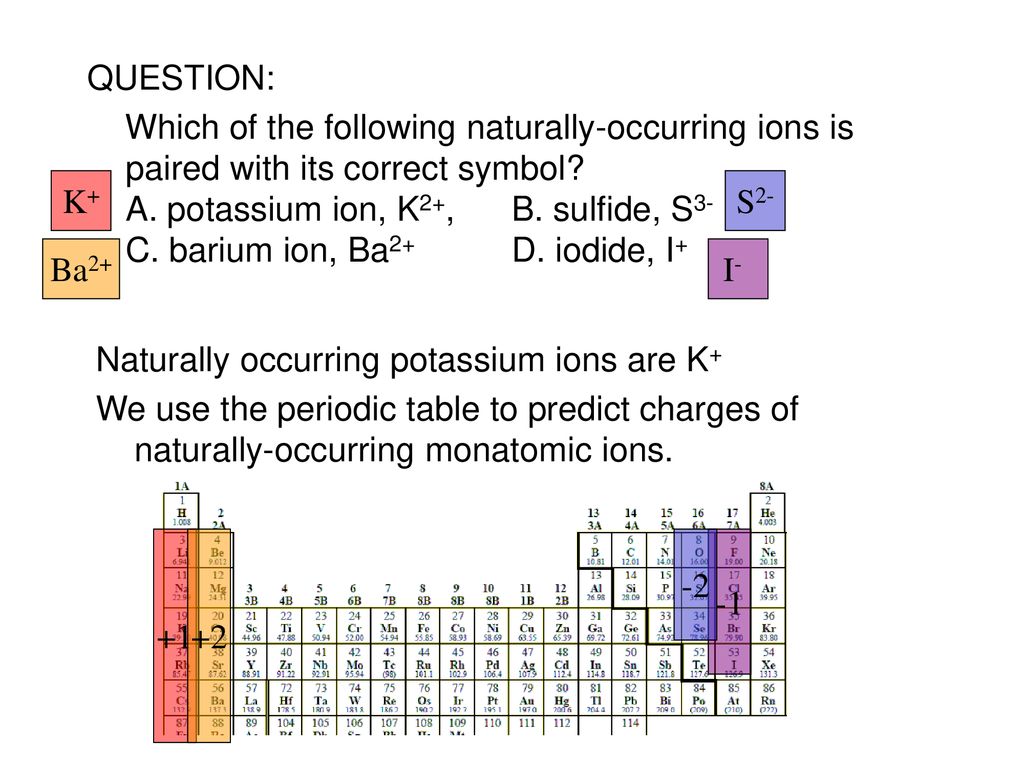 Source: slideplayer.com
Source: slideplayer.com
Using this the chemical formula of potassium oxide can be found as Potassium. Potassium was first isolated from potash the ashes of plants from which its name derives. 1 mono 2 di 3 tri 4 tetra 5 penta 6 hexa 7 hepta 8 octa 9 nona 10 deca 1 meth CH 4 methane 2 eth C2H6 ethane C2H4 ethene C2H2 ethyne 3 prop C3H8 propane C3H6 propene. Use the periodic table to find the element with an atomic number of 7. This element is nitrogen which has the symbol N.
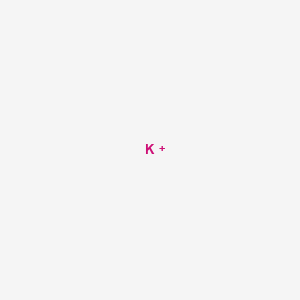
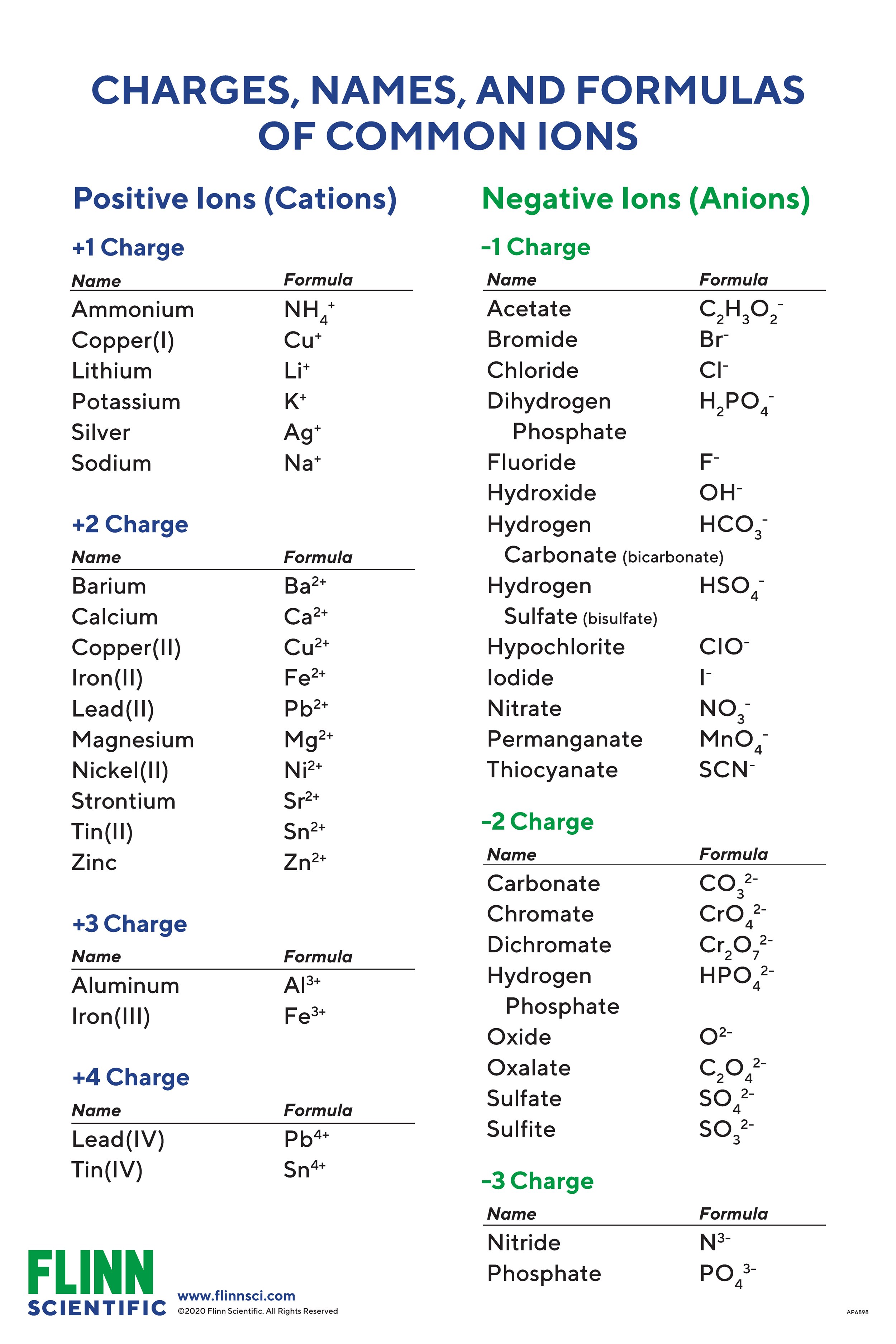
In the periodic table potassium is one of the alkali metals. Potassium is the positively-charged metal ion cation while nitride is the negatively-charged non-metal ion anion. Hence it is due a small salty herb that we now end up with the symbol K for the element pot-ash-ium. Eg H1 K19 Ur92. The chemical symbol for Potassium is K.
Write the name and symbol of the ion formed when. Since it only needs to get rid of one electron the ion will be a cation with a charge of 1 which means the symbol will be K. A potassium atom loses one electron b. Potassium nitrate is a chemical compound with the chemical formula KNO3. A zinc atom loses two electrons c.
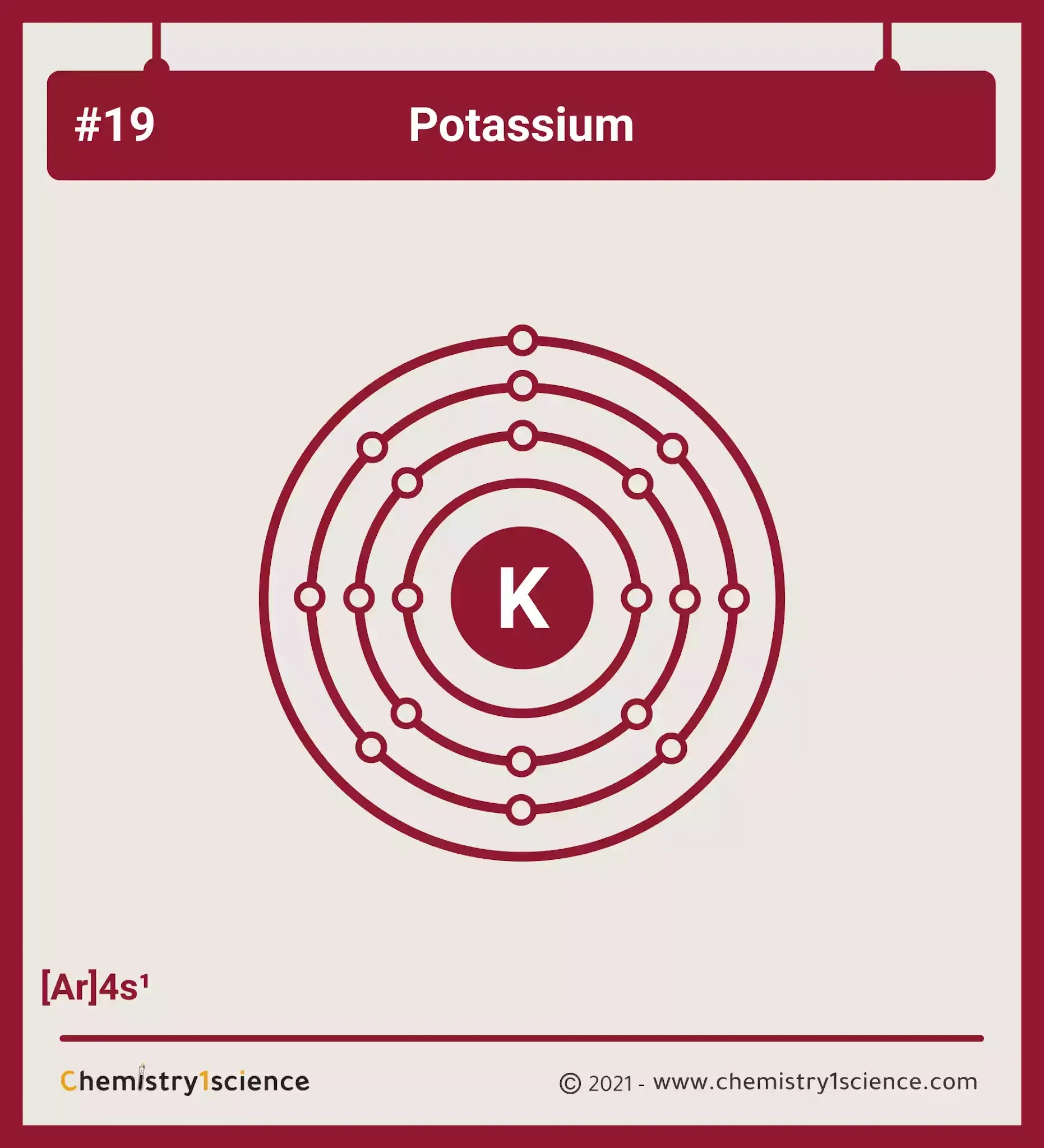 Source: chemistry1science.com
Source: chemistry1science.com
It is formed when the element chlorine gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Subscript 1 is not often written. This salt can appear as colourless crystals crystalline powder in white or white grains under standard temperature and pressure. A flourine atom gains one electron. Why does potassium have 19 electrons.

Most potassium 95 goes into fertilizers and the rest goes mainly into making potassium hydroxide KOH by the electrolysis of potassium chloride solution and then converting this to potassium carbonate K 2 CO 3. Beside above is potassium an anion. Hence it is due a small salty herb that we now end up with the symbol K for the element pot-ash-ium. A flourine atom gains one electron. Potassium is the positively-charged metal ion cation while nitride is the negatively-charged non-metal ion anion.
 Source: slideplayer.com
Source: slideplayer.com
Eg H1 K19 Ur92. What is the electron configuration of a potassium ion. In potassium sulfate the cation is potassium ion and anion is sulfate ion. A fluorine atom gains one electron Write the charge and symbol in the chart below when an atom forms an ion. Element symbol of valence e- lose or gain Symbol and Is this a cation or anion.
 Source: chegg.com
Source: chegg.com
Give the symbol of an ion that has 10 e - and 7 p. This work is done by the NaK ATPase pump which pumps 3 Na ions out of the cell and 2K into the cell to generate the Na and K concentration gradient. The second is that the membrane is semipermeable to that ion. The symbol and charge of sulfate ion is S O 4 2. Potassium K is a positively charged electrolyte cation which is present throughout the body in both intracellular and extracellular fluids.
 Source: en.wikipedia.org
Source: en.wikipedia.org
A zinc atom loses two electrons. But in order for this process to occur a concentration gradient of potassium ions must first be set up. A flourine atom gains one electron. It is always constant. A potassium atom loses one electron b.
 Source: slidetodoc.com
Source: slidetodoc.com
Is Phosphate an anion or cation. 1 mono 2 di 3 tri 4 tetra 5 penta 6 hexa 7 hepta 8 octa 9 nona 10 deca 1 meth CH 4 methane 2 eth C2H6 ethane C2H4 ethene C2H2 ethyne 3 prop C3H8 propane C3H6 propene. Eg H1 K19 Ur92. Subscript 1 is not often written. There is an ion channel that allows for the.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title potassium symbol of ion by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






