Your Periodic table definition class 10 images are ready. Periodic table definition class 10 are a topic that is being searched for and liked by netizens today. You can Find and Download the Periodic table definition class 10 files here. Find and Download all free vectors.
If you’re searching for periodic table definition class 10 pictures information related to the periodic table definition class 10 topic, you have come to the right site. Our website always gives you suggestions for downloading the maximum quality video and image content, please kindly hunt and locate more enlightening video content and images that match your interests.
Periodic Table Definition Class 10. Achievements of Mendeleevs Periodic Table Systematic study of the elements. Mendeleev laid more stress on similarity in properties rather on increasing. He left the space for corresponding elements in his periodic table which were not even discovered then. Modern Periodic Table of Elements class 10 science NCERT Modern Periodic Classification Law of Modern Periodic Table states that properties of elements are the periodic function of their atomic numbers.
 How To Learn Periodic Table Learn Cbse From learncbse.in
How To Learn Periodic Table Learn Cbse From learncbse.in
Iii Modern Periodic table has 7 periods and 18 groups. Answer 1 i The modern periodic law states that The properties of elements are the periodic functions of their atomic number ii Henry Moseley put forward the modern periodic law. The main features of modern periodic table. This made the study of the elements quite systematic. CBSE Class 10 Science Notes Chapter 5 Periodic Classification of Elements The periodic table is a tabular method of displaying the elements in such a way that the elements having similar properties occur in the same vertical column or group. What are horizontal rows and vertical.
You should always go through questions that have come in previous years.
Lavoisier divided elements into two main types known as metals and non-metals. Below which have come in past board exams. Across each row or period of the periodic table the number of valence electrons in groups 1 2 and 13 18 increases by one from one element to the next. Periodic table in full periodic table of the elements in chemistry the organized array of all the chemical elements in order of increasing atomic number ie the total number of protons in the atomic nucleus. Elements with similar properties were grouped together that made the study of their chemical and physical properties easier. This made the study of the elements quite systematic.
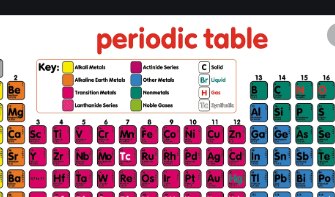 Source: icsehelp.com
Source: icsehelp.com
What is Periodic Table Definition The periodic table is a tabular arrangement of the chemical elements. It was the motivation for the discovery of some new elements. Placement of elements in Mendeleevs periodic table helped in correcting the atomic masses of certain elements. The modern periodic table is arranged in such a way that all the elements have an increasing atomic number and subsequently increasing mass number. According to this law in certain triads grout of three elements the atomic mass of the.
 Source: chemistrytalk.org
Source: chemistrytalk.org
The Periodic Table proved to be the unifying principle in chemistry. For example the atomic. What is Periodic Table Definition The periodic table is a tabular arrangement of the chemical elements. Although he was able to predict the properties of those elements through his periodic classification of elements. Earlier attempts of the classification of elements.
 Source: khanacademy.org
Source: khanacademy.org
The categorization of elements in a tabular form according to their properties became popular. CBSE Class 10 Science Notes Chapter 5 Periodic Classification of Elements The periodic table is a tabular method of displaying the elements in such a way that the elements having similar properties occur in the same vertical column or group. Introduction to Mendeleev Periodic Table. Elements in the modern periodic table are arranged in 7 periods and 18 groups. Although he was able to predict the properties of those elements through his periodic classification of elements.
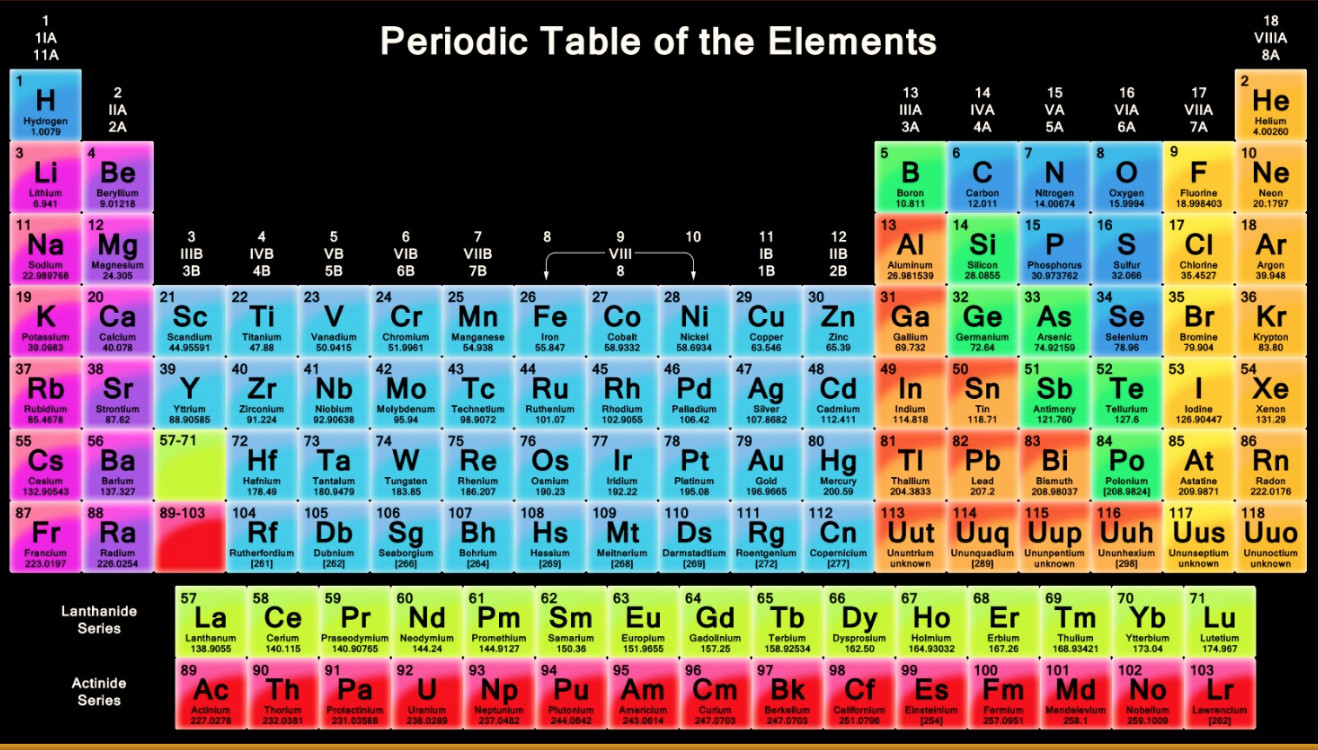 Source: classnotes.org.in
Source: classnotes.org.in
Although he was able to predict the properties of those elements through his periodic classification of elements. Classification means identifying similar species and grouping them together. Correction of atomic masses. This will help you to understand the pattern of questions in. Although he was able to predict the properties of those elements through his periodic classification of elements.
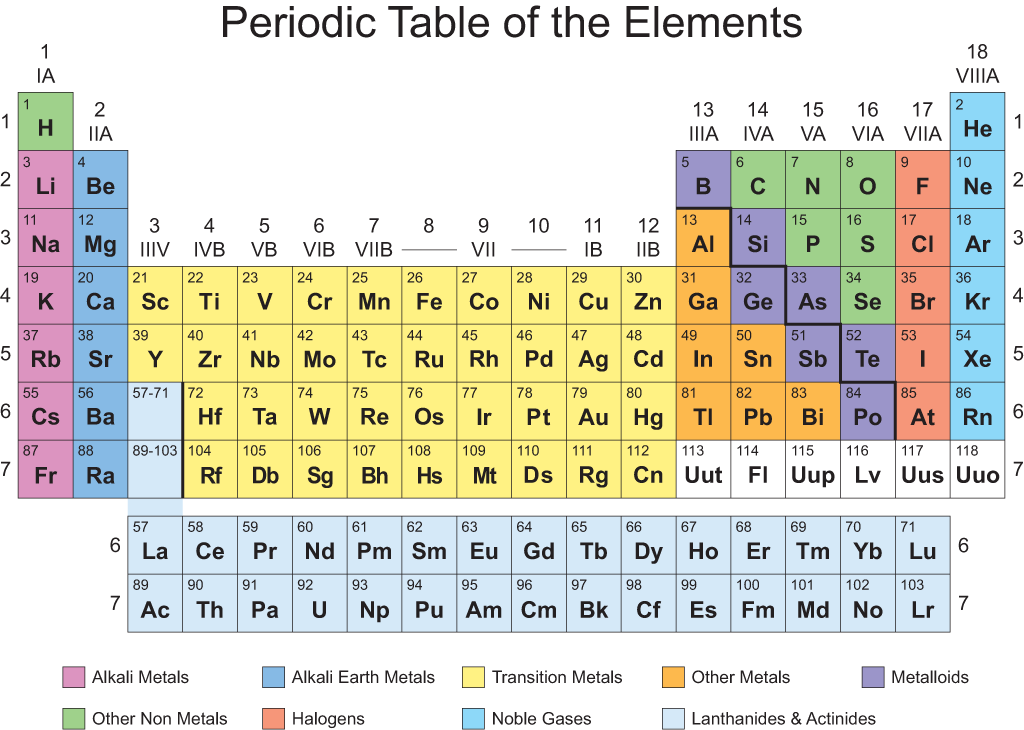 Source: successcds.net
Source: successcds.net
You should always go through questions that have come in previous years. Below which have come in past board exams. Interactive periodic table of elements - your complete guide to the elements including definition mass names of each chemical in the periodic table. Lavoisier divided elements into two main types known as metals and non-metals. Placement of elements in Mendeleevs periodic table helped in correcting the atomic masses of certain elements.
 Source: cz.pinterest.com
Source: cz.pinterest.com
Material Properties Periodic Table. This will help you to understand the pattern of questions in. Why do elements in any given group have similar properties. The Periodic Table proved to be the unifying principle in chemistry. Answer 1 i The modern periodic law states that The properties of elements are the periodic functions of their atomic number ii Henry Moseley put forward the modern periodic law.

Simply an electrolyte is a substance that can conduct an electric current when melted or dissolved in water. What does each group in the Periodic Table signify. Dobereiners Triads Newlands law of octaves. Iii Modern Periodic table has 7 periods and 18 groups. Students of ICSE Class 10 should refer to Periodic Properties and Variations of Properties ICSE Class 10 Chemistry board year questions and solutions.
 Source: toppr.com
Source: toppr.com
Elements with similar properties were grouped together that made the study of their chemical and physical properties easier. Positively charged ions are called cations. Iii How many groups and periods does modern periodic table have. Earlier attempts of the classification of elements. Material Properties Periodic Table.
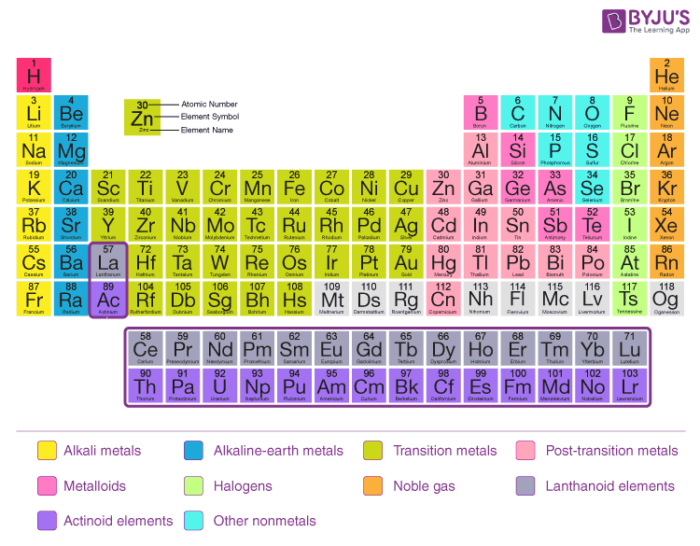 Source: byjus.com
Source: byjus.com
Dobereiners Triads Newlands law of octaves. Science Class 10 Notes for Periodic Classification of Elements 1. The tabular form structure in which various elements are arranged according to their properties is known as the periodic table. Doberiners Law of Triads. Mendeleevs Periodic Table In the year 1869 Dmitri Mendeleev arranged all 63 elements in rows or columns in order of their atomic weight.

The tabular form structure in which various elements are arranged according to their properties is known as the periodic table. According to this law in certain triads grout of three elements the atomic mass of the. What are horizontal rows and vertical. Interactive periodic table of elements - your complete guide to the elements including definition mass names of each chemical in the periodic table. The Periodic Table proved to be the unifying principle in chemistry.
 Source: learncbse.in
Source: learncbse.in
According to this law in certain triads grout of three elements the atomic mass of the. Positively charged ions are called cations. Mendeleev laid more stress on similarity in properties rather on increasing. But disadvantage is the presence of only few triads. Students of ICSE Class 10 should refer to Periodic Properties and Variations of Properties ICSE Class 10 Chemistry board year questions and solutions.
 Source: toppr.com
Source: toppr.com
Periodic table in full periodic table of the elements in chemistry the organized array of all the chemical elements in order of increasing atomic number ie the total number of protons in the atomic nucleus. The modern periodic table is arranged in such a way that all the elements have an increasing atomic number and subsequently increasing mass number. He examined the relationship between the atomic masses of the elements and their physical and chemical properties. Achievements of Mendeleevs Periodic Table Systematic study of the elements. The main features of modern periodic table.
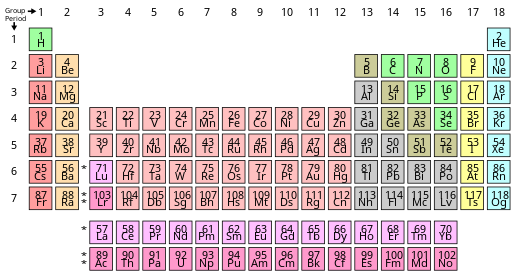 Source: wikiwand.com
Source: wikiwand.com
Iii How many groups and periods does modern periodic table have. But disadvantage is the presence of only few triads. Achievements of Mendeleevs Periodic Table Systematic study of the elements. Students of ICSE Class 10 should refer to Periodic Properties and Variations of Properties ICSE Class 10 Chemistry board year questions and solutions. He left the space for corresponding elements in his periodic table which were not even discovered then.
 Source: byjus.com
Source: byjus.com
Mendeleev laid more stress on similarity in properties rather on increasing. Across each row or period of the periodic table the number of valence electrons in groups 1 2 and 13 18 increases by one from one element to the next. Positively charged ions are called cations. An electrolyte is a substance that dissociates in water into charged particles called ions. There is a recurring pattern called the periodic law in their properties in which elements in the same column group have similar properties.
 Source: toppr.com
Source: toppr.com
Science Class 10 Notes for Periodic Classification of Elements 1. Elements are arranged in the increasing order of atomic numbers. Doberiners Law of Triads. In a tabular arrangement in which a row is a time and a column is a group they are assorted. What does each group in the Periodic Table signify.
 Source: learncbse.in
Source: learncbse.in
Dobereiners triad consider three elements in which atomic mass of central element is the arithmetic mean to two other elements. Elements in the modern periodic table are arranged in 7 periods and 18 groups. The mass number of an element is determined by the number of protons and neutrons combined. Periodic table in full periodic table of the elements in chemistry the organized array of all the chemical elements in order of increasing atomic number ie the total number of protons in the atomic nucleus. What does each group in the Periodic Table signify.
 Source: pinterest.com
Source: pinterest.com
For example each element of group I has one electron in its outermost shell. Elements are arranged in the increasing order of atomic numbers. You should always go through questions that have come in previous years. But disadvantage is the presence of only few triads. The tabular form structure in which various elements are arranged according to their properties is known as the periodic table.
 Source: byjus.com
Source: byjus.com
This will help you to understand the pattern of questions in. Prediction of new elements and their properties. Classification means identifying similar species and grouping them together. Mendeleevs Periodic Table In the year 1869 Dmitri Mendeleev arranged all 63 elements in rows or columns in order of their atomic weight. Mendeleev laid more stress on similarity in properties rather on increasing.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title periodic table definition class 10 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






