Your Periodic table atomic mass units images are available in this site. Periodic table atomic mass units are a topic that is being searched for and liked by netizens today. You can Get the Periodic table atomic mass units files here. Get all royalty-free images.
If you’re searching for periodic table atomic mass units images information connected with to the periodic table atomic mass units keyword, you have pay a visit to the ideal site. Our site frequently provides you with hints for viewing the highest quality video and picture content, please kindly search and locate more enlightening video articles and images that match your interests.
Periodic Table Atomic Mass Units. Atomic Weight amu gmol 1. Hydrogen for example is the first element. The atomic weight or relative atomic mass does not really have units but. The following article will help you to gain more information about the same.
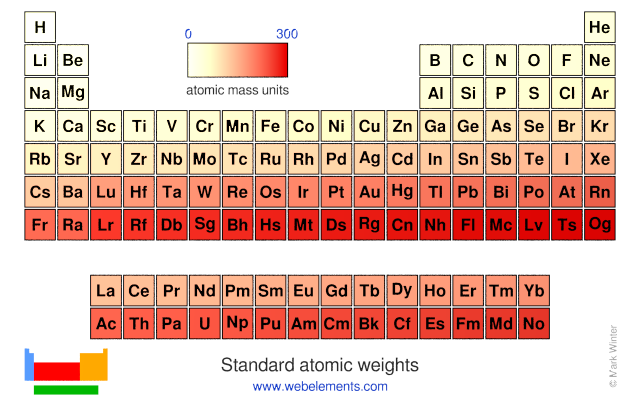 Webelements Periodic Table Periodicity Standard Atomic Weights Periodic Table Gallery From webelements.com
Webelements Periodic Table Periodicity Standard Atomic Weights Periodic Table Gallery From webelements.com
3 - Atomic Mass. A The atomic mass of Ag is 10787 amu and the molar mass of silver equals 10787 gmol. Hence it has no units. Surgeon explains at home fix for dark spots and uneven skin tones on skin. However the atomic mass on the periodic table is stated as a decimal number because it is an average of the various isotopes of an element. Atomic Weight amu gmol 1.
6 - Melting point.
The truth is that atomic weights have changed as a function of time. Since 1899 the IUPAC Commission on Isotopic Abundances and Atomic Weights CIAAW. Protons and neutrons are both hadron particles that have a positive charge or a neutral charge respectively and each are considered to have a mass of 1 atomic mass unit amu. 6 - Melting point. The truth is that atomic weights have changed as a function of time. So as you can tell as long as Avogadros hypothesis is true we can choose an atom of any element to be our standard.
 Source: study.com
Source: study.com
Individual atoms always have an integer number of atomic mass units. Atomic number - Name alphabetically. Fix your dark spots. The unit of measure for mass is the atomic mass unit amu. When the mass is expressed in AMU it roughly reflects the sum of the number of protons and neutrons in the atomic nucleus electrons have so much less.
 Source: pinterest.com
Source: pinterest.com
3 - Atomic Mass. Is the modern periodic table arranged by atomic number. This value on a periodic table is given in atomic mass units or amu but for chemistry calculations you usually write atomic mass in terms of grams per mole or gmol. Then it follows that the atomic mass of chlorine is 295 x 12 3545. A The atomic mass of Ag is 10787 amu and the molar mass of silver equals 10787 gmol.
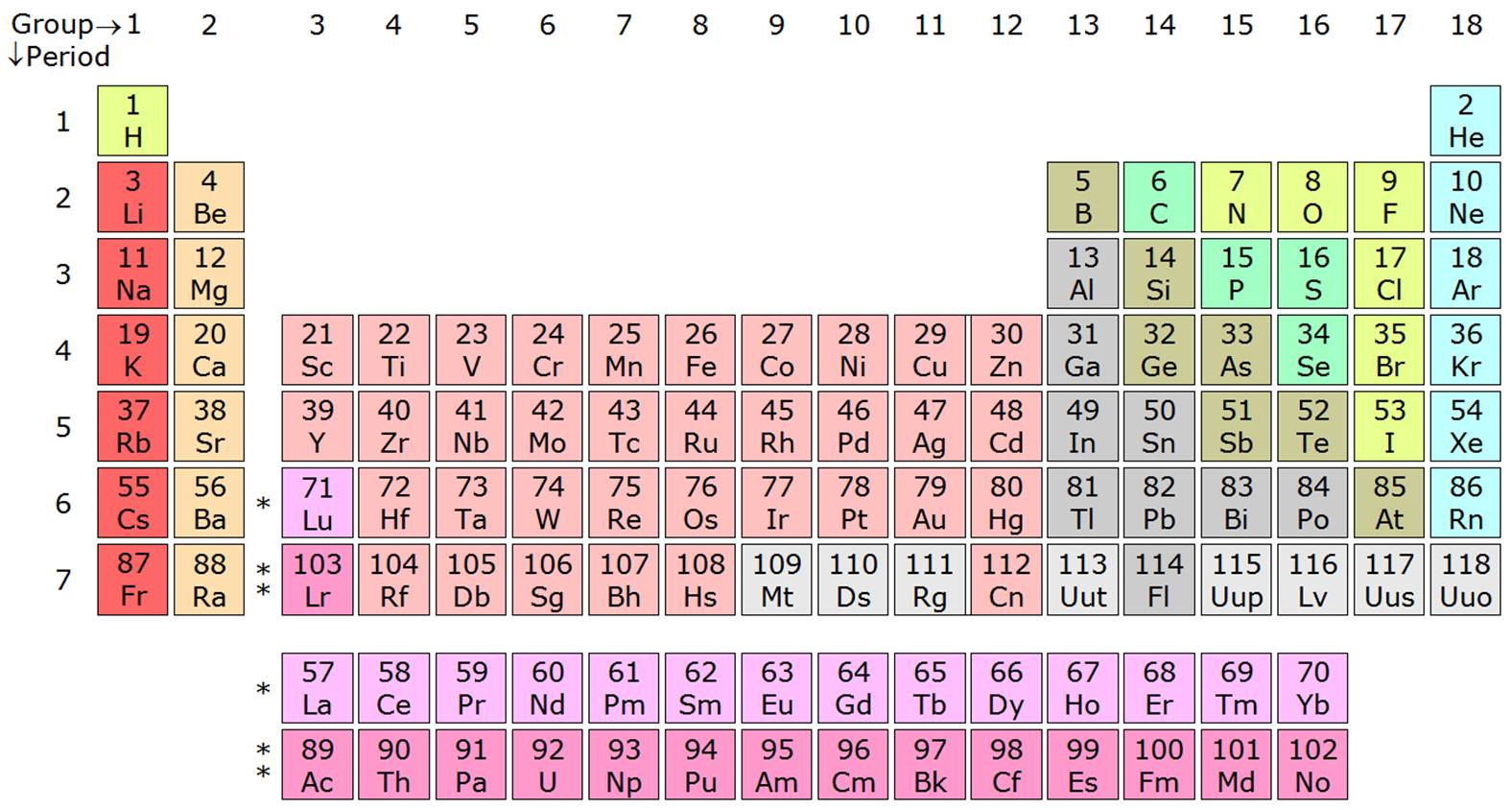 Source: chem.libretexts.org
Source: chem.libretexts.org
Besides the standard kilogram it is a second mass standard. Fix your dark spots. Periodic Table One atomic mass unit is equal to 166 x 10-24 grams. One atomic mass unit is equal to 166 x 10-24 grams. 3 is 1401 amu 3101amu 1704 amu.
 Source: sciencenotes.org
Source: sciencenotes.org
Individual atoms always have an integer number of atomic mass units. For each element the atomic weight is on the periodic table right under the symbol of the. Hence it has no units. The atomic number is simply a digit which is used for placing the elements in the periodic table. Atomic mass units are described as a unit of measurement for atoms and molecules just like the mass of a person may be expressed in pounds or kilograms.
 Source: eventos.fct.unl.pt
Source: eventos.fct.unl.pt
So clearly the atomic masses on the periodic table have no units attached to them because they are not. Only isotopes of an element share the same atomic number. Hydrogen for example is the first element. Standard Atomic Weight The standard atomic weight is the average mass of an element in atomic mass units amu. The following article will help you to gain more information about the same.
![]() Source: shutterstock.com
Source: shutterstock.com
The molar mass equals the sum of the atomic masses expressed in gmol. 1 - Atomic number. Hydrogen for example is the first element. One unified atomic mass unit can be quantified as the weight of one-twelfth of the mass of a. The truth is that atomic weights have changed as a function of time.
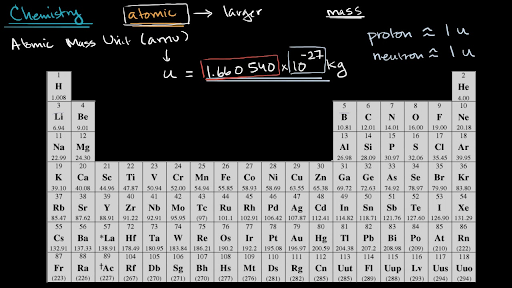
Amus are units of. It is a decimal number due to the fact that there are different isotopes for each element and the atomic weight is a weighted average of all the isotopes for that element and their relative abundance. Besides the standard kilogram it is a second mass standard. Atomic number of elements. Though individual atoms always have an integer number of atomic mass units the atomic mass on the periodic table is stated as a decimal number because it is an average of the various isotopes of an element.
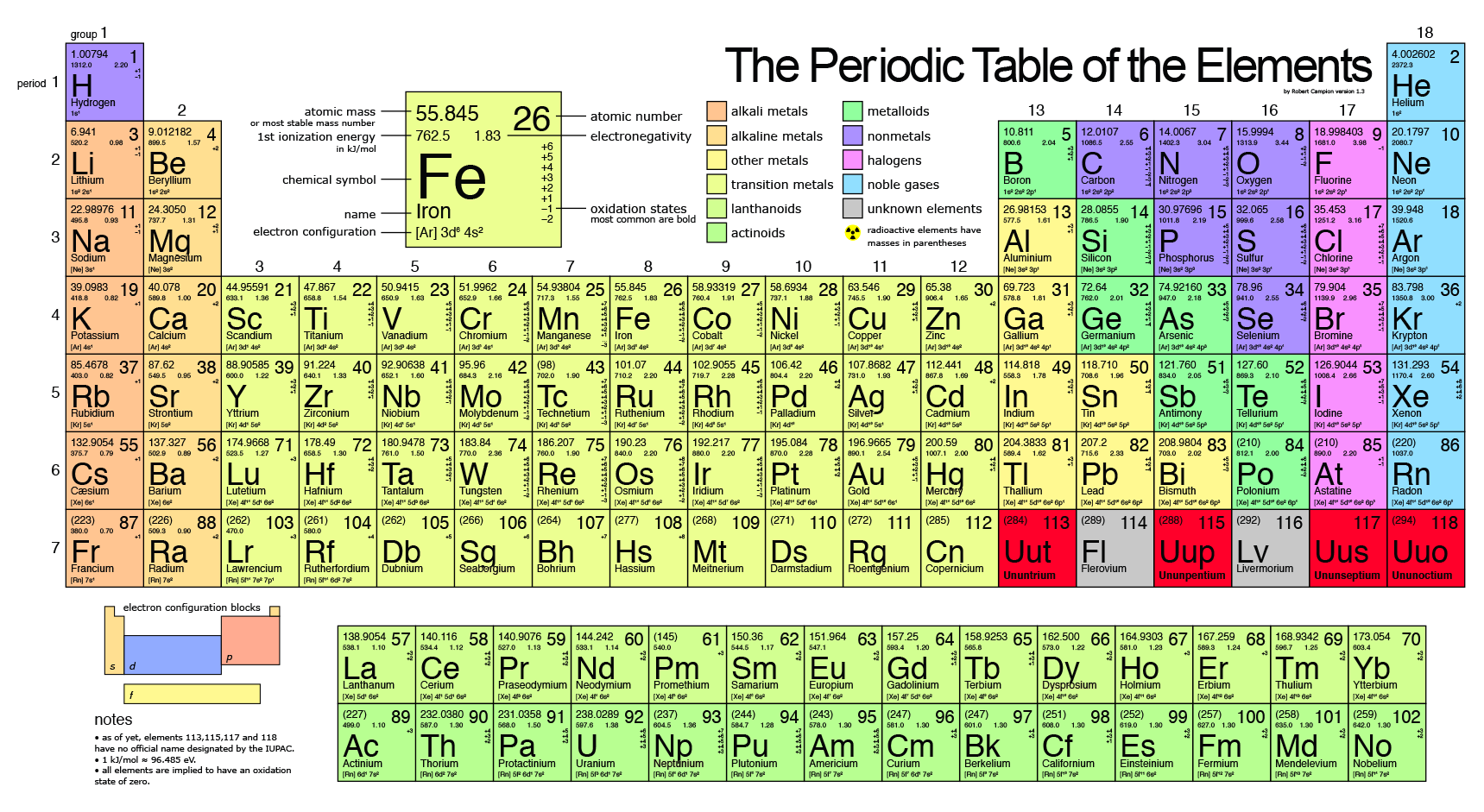 Source: socratic.org
Source: socratic.org
Relative atomic mass is the mass of an atom of an element on a scale where the mass of a carbon atom is exactly 12 units. 1 - Atomic number. We begin by finding the atomic mass of each element in the periodic table. A The atomic mass of Ag is 10787 amu and the molar mass of silver equals 10787 gmol. The unit mass for an atom element on the periodic table is not a whole number.

However the atomic mass on the periodic table is stated as a decimal number because it is an average of the various isotopes of an element. In chemistry an atomic mass unit or AMU is a physical constant equal to one-twelfth of the mass of an unbound atom of carbon-12. 115 rows Atomic Number. Atomic number of elements. A The atomic mass of Ag is 10787 amu and the molar mass of silver equals 10787 gmol.

The molar mass of ammonia. One atomic mass unit is equal to 166 x 10-24 grams. Periodic Table Atomic Mass Numbers 1 Periodic Table Atomic Mass Numbers Atomic Number Z number of protons in the nucleus also number of electrons in the neutral atom Mass Number A total number of protons plus neutrons in the nucleus Therefore A Z N where N number of neutrons A - Z 2 The General Atom. Surgeon explains at home fix for dark spots and uneven skin tones on skin. 3 is 1401 amu 3101amu 1704 amu.
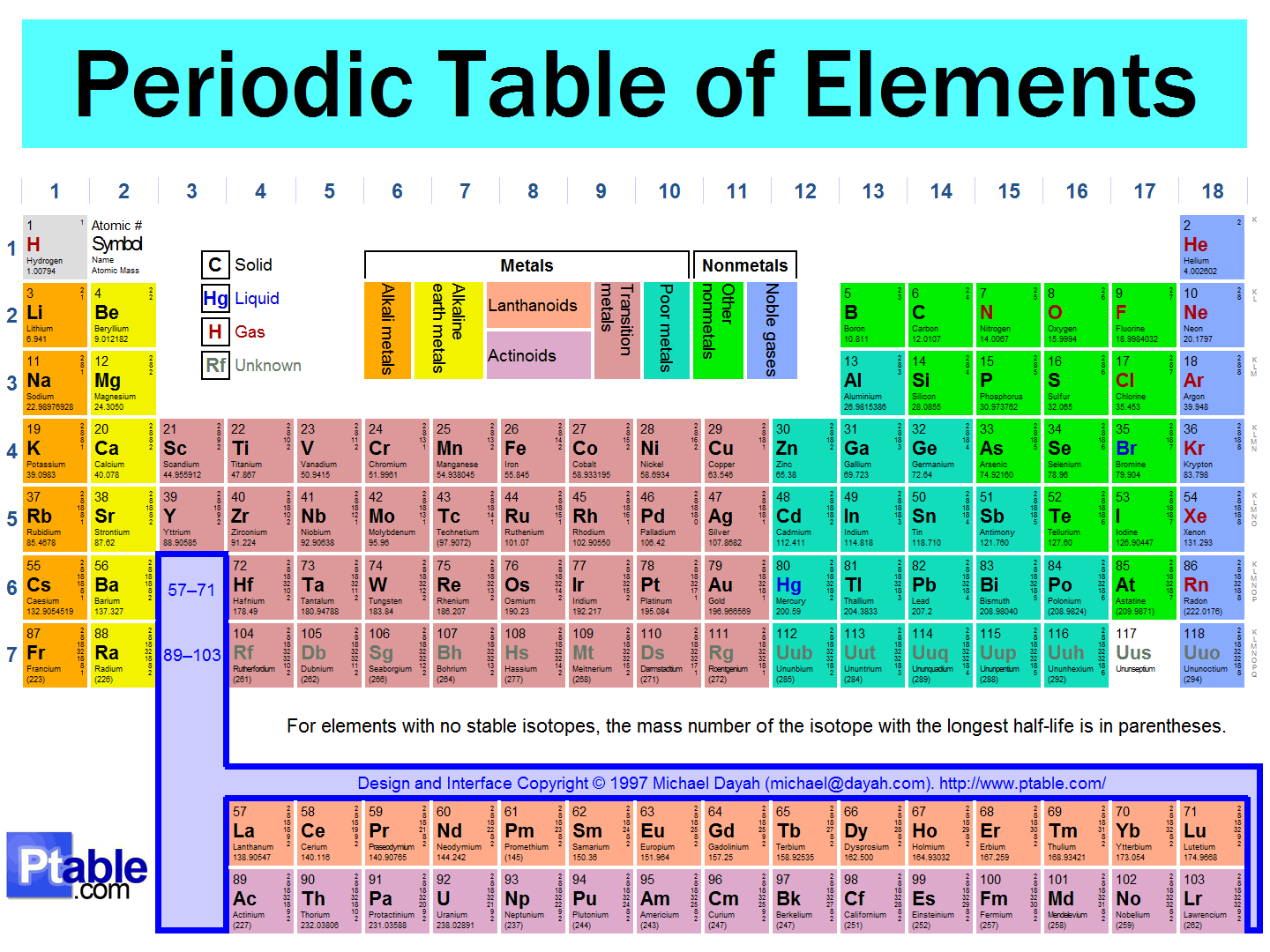 Source: socratic.org
Source: socratic.org
For each element the atomic weight is on the periodic table right under the symbol of the. One atomic mass unit is equal. The average number of neutrons for an element. Atomic weights found within a periodic table one might think are constant. Periodic Table One atomic mass unit is equal to 166 x 10-24 grams.
 Source: utedzz.blogspot.com
Source: utedzz.blogspot.com
Hydrogen for example is the first element. Atoms of the same element with different numbers of neutrons and hence different mass numbers are called isotopes of. Only isotopes of an element share the same atomic number. Atomic weights found within a periodic table one might think are constant. 1 - Atomic number.
 Source: webelements.com
Source: webelements.com
However the atomic mass on the periodic table is stated as a decimal number because it is an average of the various isotopes of an element. However the atomic mass on the periodic table is stated as a decimal number because it is an average of the various isotopes of an element. A The atomic mass of Ag is 10787 amu and the molar mass of silver equals 10787 gmol. 3 - Atomic Mass. 2 Sum of Protons and Neutrons for a Single Atom.
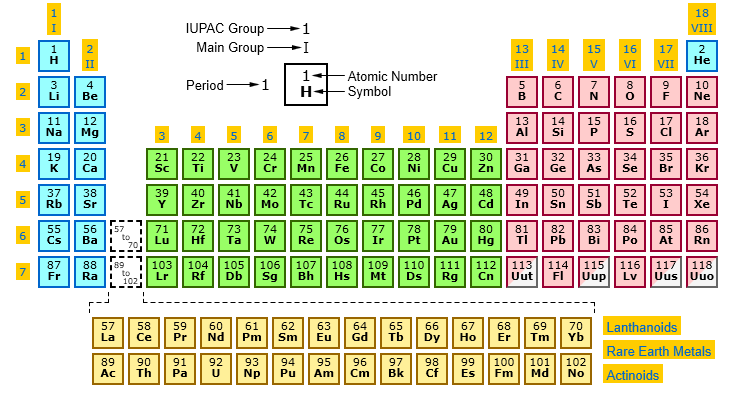 Source: periodictable.me
Source: periodictable.me
Only isotopes of an element share the same atomic number. Relative atomic mass is the mass of an atom of an element on a scale where the mass of a carbon atom is exactly 12 units. Atomic number of chemical elements in chemistry is the number of protons of an atom by which the elements are arranged in the periodic tableThe modern periodic system is formed on the basis of atomic number and electronic configuration of the atom but Mendeleev classification is based on the atomic weight or mass of an element. Though individual atoms always have an integer number of atomic mass units the atomic mass on the periodic table is stated as a decimal number because it is an average of the various isotopes of an element. Besides the standard kilogram it is a second mass standard.
 Source: photographieetpartage.org
Source: photographieetpartage.org
119 rows the periodic chart sorted by. Though individual atoms always have an integer number of atomic mass units the atomic mass on the periodic table is stated as a decimal number because it is an average of the various isotopes of an element. The periodic table provides us with a comprehensive view of the various elements. Periodic Table One atomic mass unit is equal to 166 x 10-24 grams. So clearly the atomic masses on the periodic table have no units attached to them because they are not.
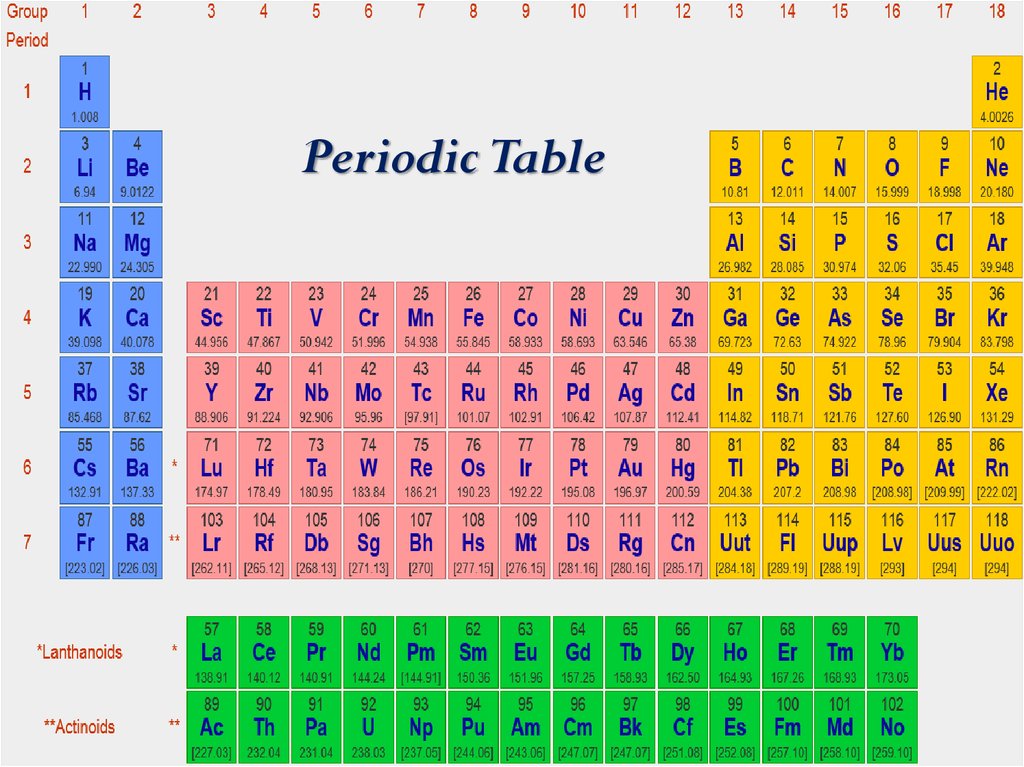 Source: ppt-online.org
Source: ppt-online.org
One unified atomic mass unit can be quantified as the weight of one-twelfth of the mass of a. Though individual atoms always have an integer number of atomic mass units the atomic mass on the periodic table is stated as a decimal number because it is an average of the various isotopes of an element. The following article will help you to gain more information about the same. 115 rows Atomic Number. The truth is that atomic weights have changed as a function of time.

115 rows Atomic Number. The relation between the two units is. 3 - Atomic Mass. Individual atoms always have an integer number of atomic mass units. A The atomic mass of Ag is 10787 amu and the molar mass of silver equals 10787 gmol.

So as you can tell as long as Avogadros hypothesis is true we can choose an atom of any element to be our standard. The truth is that atomic weights have changed as a function of time. 2 Sum of Protons and Neutrons for a Single Atom. 6 - Melting point. Along with atomic mass and atomic number values this table helps us to understand the various properties abbreviations and names of all the elements present in nature.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title periodic table atomic mass units by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






