Your Modern periodic table law images are ready in this website. Modern periodic table law are a topic that is being searched for and liked by netizens today. You can Download the Modern periodic table law files here. Download all royalty-free images.
If you’re looking for modern periodic table law pictures information connected with to the modern periodic table law interest, you have visit the ideal blog. Our site always provides you with suggestions for seeking the maximum quality video and picture content, please kindly search and find more informative video content and graphics that fit your interests.
Modern Periodic Table Law. Modern periodic law states that if the elements are arranged in tabular form in the increasing order of their atomic numbers then the properties of the elements are repeated after definite regular intervals or periods. The columns are called as groups. The modern periodic law states that the physical and chemical properties of the elements are the periodic function of the atomic numbers and electronic configurations. The modern statement of the periodic law is that the properties of the elements are the periodic function of their atomic numbers.
 The Modern Periodic Table Video Khan Academy From khanacademy.org
The Modern Periodic Table Video Khan Academy From khanacademy.org
The modern periodic table is the present form of the periodic table. Regarding the modern periodic law the elements were arranged to increase their atomic numbers to form the modern periodic table. Modern periodic law states that if the elements are arranged in tabular form in the increasing order of their atomic numbers then the properties of the elements are repeated after definite regular intervals or periods. These Modern Periodic Law and the Present Form of the Periodic Table MCQs will help you to prepare for any competitive exams like. The columns are called as groups. Modern periodic law of element may be defined as the physical and chemical properties of the elements are periodic functions of their atomic numbers.
This table is based on the Modern periodic Law given by Henry Moseley.
Regarding the modern periodic law the elements were arranged to increase their atomic numbers to form the modern periodic table. The table is the arrangement of elements in increasing order of their atomic numbers. The periodic table continues to evolve with the progress of science. This table is based on the Modern periodic Law given by Henry Moseley. The modern periodic table is developed after the periodic law and a periodic table given by Mendeleev. A The modern periodic law states that the properties of elements are a periodic function of their atomic numbers.
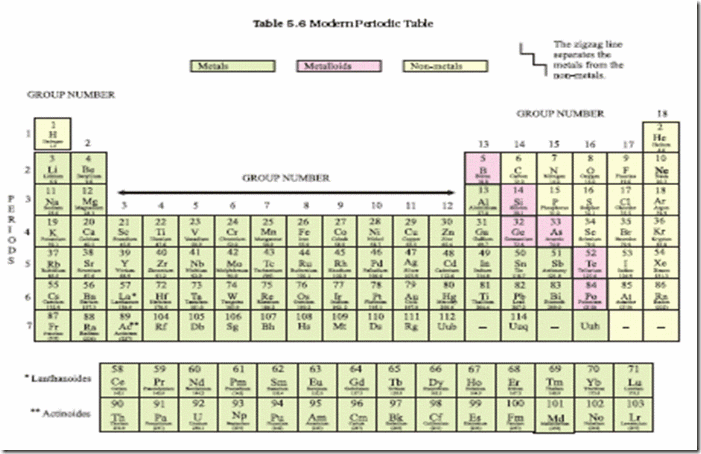 Source: cbse.myindialist.com
Source: cbse.myindialist.com
NEET AIIMS JEE Mains JEE Advance IIT JEE JIPMER and other Exams at all levels you just have to practice regularly. What are the advantages of the modern periodic table. These Modern Periodic Law and the Present Form of the Periodic Table MCQs will help you to prepare for any competitive exams like. Modern Periodic Table is the arrangement of all known elements in the increasing order of their ATOMIC NUMBERS. Thus the modern periodic law can be stated as.
 Source: funscience.in
Source: funscience.in
The elements having same number of valence. Modern Periodic Table is the arrangement of all known elements in the increasing order of their ATOMIC NUMBERS. The noble gases are not. B The modern periodic law states that a no two electrons with the same spin can be found in the same place in an atom. Modern Periodic Law and the Present Form of the Periodic Table MCQs 1.
 Source: knowitinfo.com
Source: knowitinfo.com
In nature only elements up to atomic number 94 exist. In the modern periodic table atoms with similar electron configurations are placed in the same column. Modern periodic law states that if the elements are arranged in tabular form in the increasing order of their atomic numbers then the properties of the elements are repeated after definite regular intervals or periods. These Modern Periodic Law and the Present Form of the Periodic Table MCQs will help you to prepare for any competitive exams like. The arrangement of the periodic table is now based on how electrons fill various energy levels 3.
 Source: khanacademy.org
Source: khanacademy.org
The elements in a period have consecutive atomic numbers. According to this law if we arrange the elements on basis of their increasing atomic numbers we find that the elements with similar physical and chemical behavior will be grouped together after a certain interval of elements. B When elements are arranged according to increasing atomic numbers there is a periodicity in the electronic configurations of the elements. Modern periodic law states that if the elements are arranged in tabular form in the increasing order of their atomic numbers then the properties of the elements are repeated after definite regular intervals or periods. Modern Periodic Law states that Physical and chemical properties of the elements are the periodic function of their atomic number.
 Source: knowitinfo.com
Source: knowitinfo.com
The chemical and physical properties of elements are periodic functions of their ATOMIC NUMBERS. What is the modern periodic table law. The elements with the similar properties repeat after certain regular intervals. In the modern periodic table atoms with similar electron configurations are placed in the same column. The periodic law states When elements are arranged in order of increasing atomic number there is a periodic repetition of their chemical and physical properties Practice Use the link below to answer the following questions.
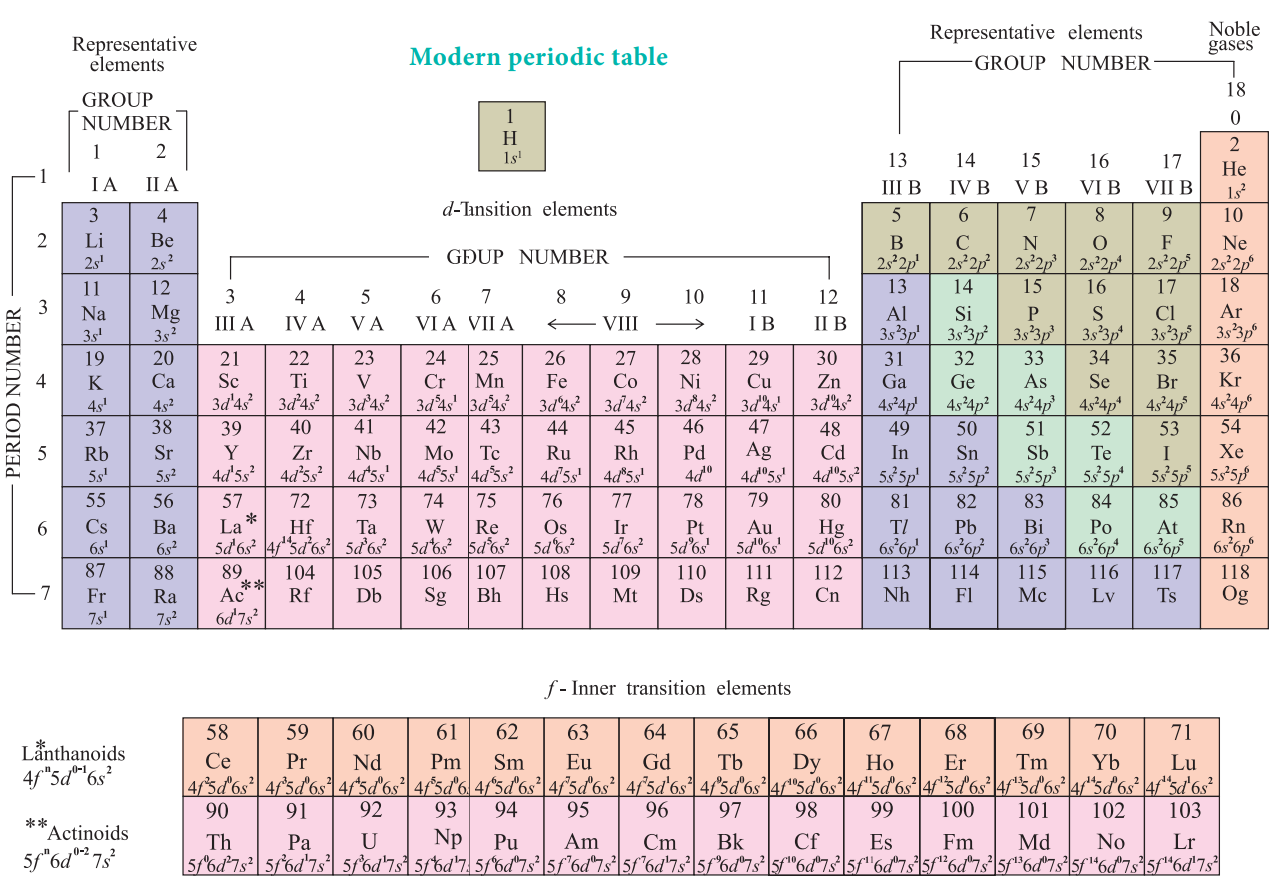 Source: brainkart.com
Source: brainkart.com
What are the advantages of the modern periodic table. The Modern periodic law states The chemical and physical properties of elements are a periodic function of their atomic numbers. The arrangement of the periodic table is now based on how electrons fill various energy levels 3. The chemical and physical properties of elements are the periodic functions of their atomic numbers and electronic configurations The modern long form of periodic table was constructed based on above law. Modern Periodic Table is the arrangement of all known elements in the increasing order of their ATOMIC NUMBERS.
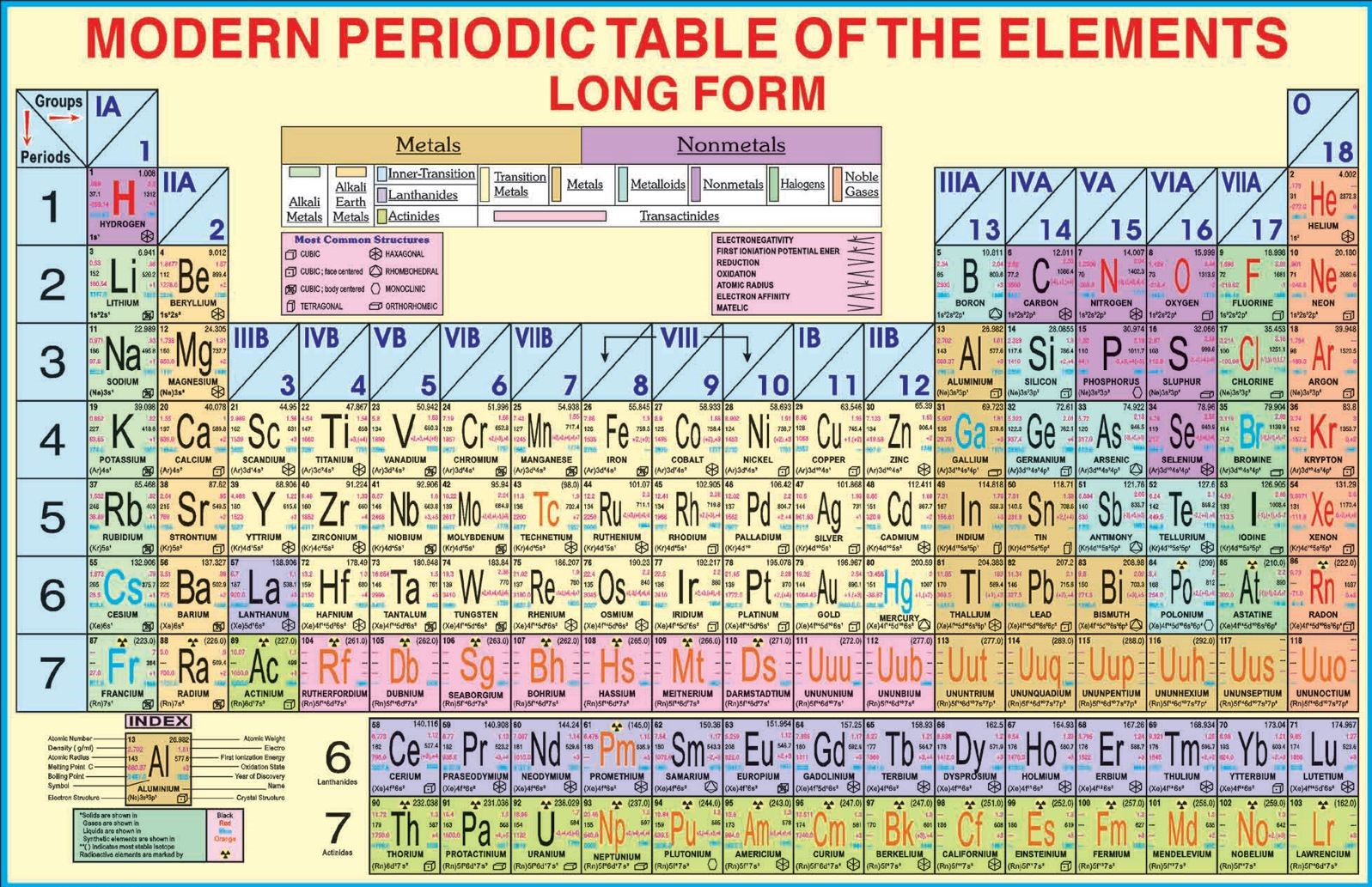 Source: onlinesciencenotes.com
Source: onlinesciencenotes.com
Elements across in periods show integral increase in valence electrons. What is the modern periodic table law. The modern periodic table is developed after the periodic law and a periodic table given by Mendeleev. The elements with the similar properties repeat after certain regular intervals. B The modern periodic law states that a no two electrons with the same spin can be found in the same place in an atom.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
The periodic table continues to evolve with the progress of science. Modern Periodic Table 1. The modern statement of the periodic law is that the properties of the elements are the periodic function of their atomic numbers. Regarding the modern periodic law the elements were arranged to increase their atomic numbers to form the modern periodic table. Similar properties of the elements occur periodically when arranged by their atomic numbers not masses 2.
 Source: shaalaa.com
Source: shaalaa.com
A The modern periodic law states that the properties of elements are a periodic function of their atomic numbers. Thus the modern periodic law can be stated as. The arrangement of the periodic table is now based on how electrons fill various energy levels 3. To study the modern periodic table. In nature only elements up to atomic number 94 exist.
 Source: brainkart.com
Source: brainkart.com
The periodic table continues to evolve with the progress of science. Modern periodic law states that if the elements are arranged in tabular form in the increasing order of their atomic numbers then the properties of the elements are repeated after definite regular intervals or periods. Modern periodic law of element may be defined as the physical and chemical properties of the elements are periodic functions of their atomic numbers. Elements of the periodic table are arranged in order of increasing atomic number. What are the advantages of the modern periodic table.
 Source: researchgate.net
Source: researchgate.net
The Modern periodic law states The chemical and physical properties of elements are a periodic function of their atomic numbers. Mendeleev arranged all the 63 elements known at that time in the form of a table known as Mendeleevs periodic table. The elements with the similar properties repeat after certain regular intervals. The periodic law states When elements are arranged in order of increasing atomic number there is a periodic repetition of their chemical and physical properties Practice Use the link below to answer the following questions. Regarding the modern periodic law the elements were arranged to increase their atomic numbers to form the modern periodic table.
 Source: netexplanations.com
Source: netexplanations.com
The noble gases are not. By choosing atomic number as the basis of classification the anomalies in the original Mendeefs periodic table got removed as described below. NEET AIIMS JEE Mains JEE Advance IIT JEE JIPMER and other Exams at all levels you just have to practice regularly. Elements of the periodic table are arranged in order of increasing atomic number. To study the modern periodic table.
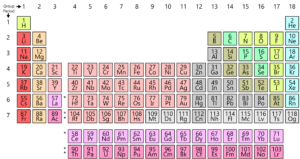 Source: toppr.com
Source: toppr.com
The arrangement of the periodic table is now based on how electrons fill various energy levels 3. Elements across in periods show integral increase in valence electrons. How did atomic number prove mass better basis of classification. In the latter part of the 18th century Mendeleev made his periodic table. It is the table of chemical elements arranged in order of atomic number such that elements with similar atomic structure appear in the vertical columns.
 Source: unacademy.com
Source: unacademy.com
Horizontal row of elements by increasing atomic number. To go further it was necessary to synthesise new elements in. Modern Periodic Law and the Present Form of the Periodic Table MCQs 1. The elements with the similar properties repeat after certain regular intervals. The modern statement of the periodic law is that the properties of the elements are the periodic function of their atomic numbers.
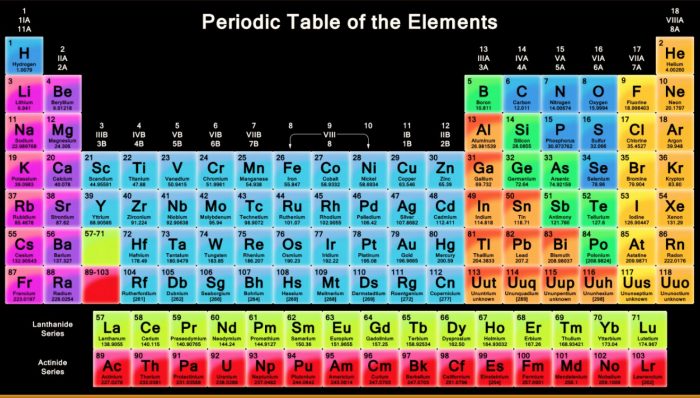 Source: classnotes.org.in
Source: classnotes.org.in
The periodic law states When elements are arranged in order of increasing atomic number there is a periodic repetition of their chemical and physical properties Practice Use the link below to answer the following questions. What is Modern Periodic Law. The modern statement of the periodic law is that the properties of the elements are the periodic function of their atomic numbers. And it consists of 18 vertical columns and 7 horizontal rows. In the modern periodic table the electronegativity of elements increases across a period row and decreases down a group column.
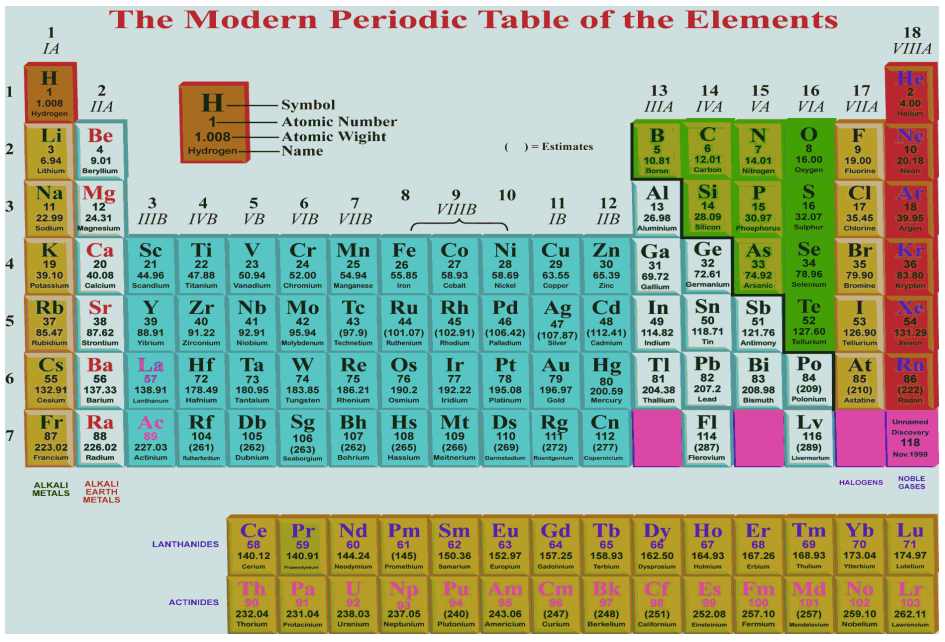 Source: aplustopper.com
Source: aplustopper.com
The periodic table and law are now a central and indispensable part of modern chemistry. Modern periodic law states that if the elements are arranged in tabular form in the increasing order of their atomic numbers then the properties of the elements are repeated after definite regular intervals or periods. What is the modern periodic table law. According to this law if we arrange the elements on basis of their increasing atomic numbers we find that the elements with similar physical and chemical behavior will be grouped together after a certain interval of elements. The chemical and physical properties of elements are periodic functions of their ATOMIC NUMBERS.
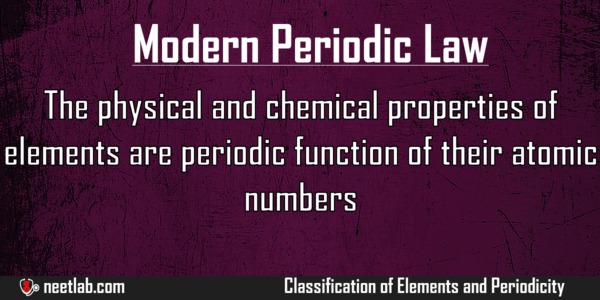 Source: neetlab.com
Source: neetlab.com
Elements of the periodic table are arranged in order of increasing atomic number. Elements of the periodic table are arranged in order of increasing atomic number. Similar properties of the elements occur periodically when arranged by their atomic numbers not masses 2. B the physical and chemical properties of an element are functions of its atomic number. The periodic table and law are now a central and indispensable part of modern chemistry.
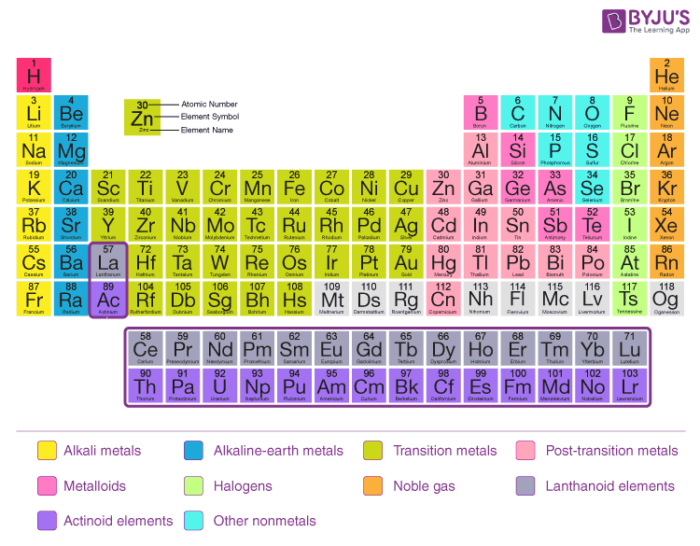 Source: byjus.com
Source: byjus.com
Elements across in periods show integral increase in valence electrons. What is the modern periodic table law. This repetition occurs if the arrangement of the elements is in order of their increasing atomic. Answer 1 of 7. The periodic table and law are now a central and indispensable part of modern chemistry.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title modern periodic table law by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






