Your How many electrons does potassium lose images are ready. How many electrons does potassium lose are a topic that is being searched for and liked by netizens now. You can Find and Download the How many electrons does potassium lose files here. Find and Download all free vectors.
If you’re searching for how many electrons does potassium lose pictures information connected with to the how many electrons does potassium lose topic, you have pay a visit to the right site. Our site frequently provides you with hints for refferencing the maximum quality video and picture content, please kindly hunt and locate more informative video articles and graphics that fit your interests.
How Many Electrons Does Potassium Lose. For the number of protons it is equal to the atomic number. All Group 1 atoms can lose one electron to form positively charged ions. Group 2 atoms lose two electrons to form positively charged ions. The name of a metal ion is the same as the name of the metal atom from which it.
 Electrons Atomic Model Project123 From sites.google.com
Electrons Atomic Model Project123 From sites.google.com
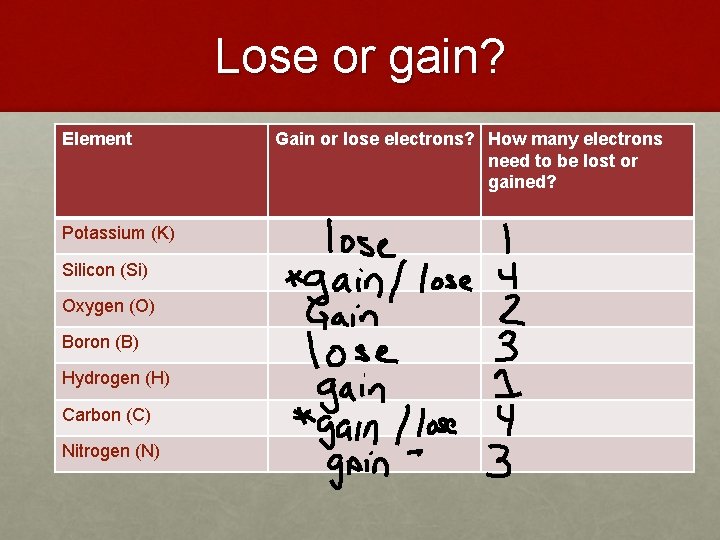
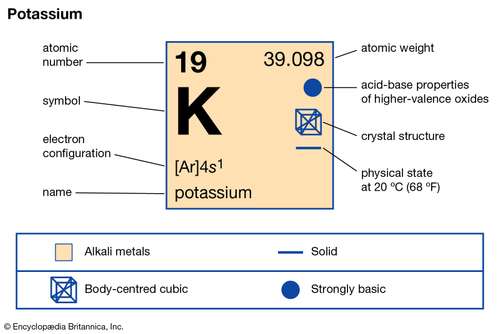
You can look up the atomic number for any element on the Periodic Table of the Elements or any Table of the Elements. This electron is transferred to a chlorine atom to form a chloride ion. The book uses Potassium as an example. That is the potassium K atom has a total of nineteen electrons. What is the charge of a potassium ion with 19 protons and 18 electrons. 18 electrons the normal 19 electrons of a potassium atom minus one because the species has a positive charge that is has lost one electron.
Potassium is in the first column and therefore has 1 electron in its outermost shell.
When potassium forms a compound with iodine potassium loses one electron from its fourth level and the third level becomes a complete outer level. Why does potassium have 18 electrons. When potassium forms a compound with iodine potassium loses one electron from its fourth level and the third level becomes a complete outer level. Yes calciumis defined as a metal because of both its physical and chemical traits. Potassium is in the first column and therefore has 1 electron in its outermost shell. All Group 1 atoms can lose one electron to form positively charged ions.
 Source: slidetodoc.com
Source: slidetodoc.com
It instead has 19 protons and 18 electrons yielding a net positive charge. The name of a metal ion is the same as the name of the metal atom from which it. Electrons equal to protons are located in a circular shell orbit outside the nucleus. It would tend to lose one electron and form a 1 ion. The atomic number is the number of protons.
 Source: itc.gsw.edu
Source: itc.gsw.edu
For example potassium atoms do this to form ions with the same electron configuration as the noble gas argon. Either atoms gain enough electrons to have eight electrons in the valence shell and become the. How many electrons does potassium gain or lose. It would tend to lose one electronand form a 1 ion. When potassium forms a compound with iodine potassium loses one electron from its fourth level and the third level becomes a complete outer level.

Electrons equal to protons are located in a circular shell orbit outside the nucleus. For the most part transition metals are an exception. In KCl both ions have full outer energy levels noble gas configuration. In Section 91 Lewis Electron Dot Diagrams we saw how ions are formed by losing electrons to make cations or by gaining electrons to form anionsThe astute reader may have noticed something. How many electrons does potassium need to lose to become an ion.

Potassium is in the first column and therefore has 1 electronin its outermost shell. Here is the generic procedure. Electrons equal to protons are located in a circular shell orbit outside the nucleus. They all have an outer shell with two electrons and are very reactive. When atoms lose or gain electrons they become what are called ionsAtoms that can lose two electrons such as Calcium can form molecules with two chemical bonds.
 Source: slideplayer.com
Source: slideplayer.com
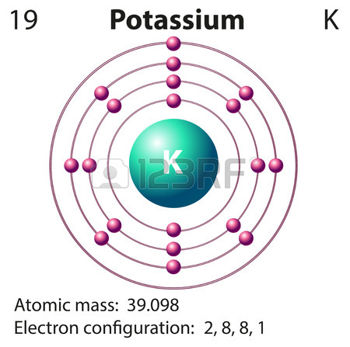
You can look up the atomic number for any element on the Periodic Table of the Elements or any Table of the Elements. Answer 1 of 3. Here is the generic procedure. Yes calciumis defined as a metal because of both its physical and chemical traits. That is the number of protons in the potassium K is 19.
 Source: sites.google.com
Source: sites.google.com
It instead has 19 protons and 18 electrons yielding a net positive charge. 1 only because it is a group 1 element so it has only 1 electron in its outer most shell and when it loses that electron it becomes stable and gets a charge of 1 on it. Therefore it only needs to lose one electron in order for. That is the number of protons in the potassium K is 19. For the number of protons it is equal to the atomic number.
 Source: socratic.org
Source: socratic.org
So potassium has won Valence Electron in Sulphur House 6123456 Right So high Eddie come Bones or form which is gonna be your product is theres this rule called the Doctor rule in which all elements 18 electrons around that and are to form a stable compound. How many electrons does potassium need to lose to become an ion. Electrons equal to protons are located in a circular shell orbit outside the nucleus. 18 electrons However the potassium ion has lost its valence electron and has therefore formed a positive action K. It would tend to lose one electronand form a 1 ion.
 Source: slideplayer.com
Source: slideplayer.com
For example potassium atoms do this to form ions with the same electron configuration as the noble gas argon. For example potassium atoms do this to form ions with the same electron configuration as the noble gas argon. How many electrons does potassium gain or lose. What is the valence of calcium. When atoms lose or gain electrons they become what are called ionsAtoms that can lose two electrons such as Calcium can form molecules with two chemical bonds.
 Source: periodictable.me
Source: periodictable.me
Mar 16 2016. The book uses Potassium as an example. All Group 1 atoms can lose one electron to form positively charged ions. The electron configuration of neutral K is 1s22s22p63s23p64s1 but in K it loses one electron so it has a new electron configuration of 1s22s22p63s23p6 means K has only 26 8 outermost electrons which makes it stable. Most atoms lose or gain just a few negatively charged electrons from their surroundings but not manganese.
 Source: sciencecoverage.com
Source: sciencecoverage.com
In transition metal atoms and in many heavier elements the octet rule does not apply. A potassium atom loses 1 electron to for a K ion single and a sulphur atom gains 2 to form S2-. For the most part transition metals are an exception. All Group 1 atoms can lose one electron to form positively charged ions. How many electrons does potassium ion K have.
 Source: sciencecoverage.com
Source: sciencecoverage.com
All Group 1 atoms can lose one electron to form positively charged ions. Therefore Potassium wants to lose one electron. For example potassium atoms do this to form ions with the same electron configuration as the noble gas argon. What is the valence of calcium. In KCl both ions have full outer energy levels noble gas configuration.
 Source: periodictable.me
Source: periodictable.me
In transition metal atoms and in many heavier elements the octet rule does not apply. Therefore it only needs to lose one electron in order for. The atomic number is the number of protons. Group of answer choices. What is the valence of calcium.
 Source: youtube.com
Source: youtube.com
Here is the generic procedure. Either atoms gain enough electrons to have eight electrons in the valence shell and become the. Does potassium lose an electron. What happens when aluminum becomes an ion. How many electrons does potassium need to lose to become an ion.

How many electrons does potassium shown above need to gain or lose to become stable. Therefore it only needs to lose one electron in order for. How many electrons does potassium shown above need to gain or lose to become stable. 18 electrons the normal 19 electrons of a potassium atom minus one because the species has a positive charge that is has lost one electron. What is the charge of a potassium ion with 19 protons and 18 electrons.

1 only because it is a group 1 element so it has only 1 electron in its outer most shell and when it loses that electron it becomes stable and gets a charge of 1 on it. What is the valence of calcium. Therefore it only needs to lose one electron in order for. Why does potassium have 18 electrons. How many electrons does potassium gain or lose.

What is the name and symbol for the cation formed when a potassium atom loses one electron. Therefore Potassium wants to lose one electron. If you know the elements name the Table will tell you. Here is the generic procedure. Does potassium lose an electron.

Therefore Potassium wants to lose one electron. The name of a metal ion is the same as the name of the metal atom from which it. When potassium forms a compound with iodine potassium loses one electron from its fourth level and the third level becomes a complete outer level. Therefore it only needs to lose one electron in order for. For the most part transition metals are an exception.
 Source: slideshare.net
Source: slideshare.net
Potassium is in the first column and therefore has 1 electron in its outermost shell. That is the number of protons in the potassium K is 19. Many of the ions that form have eight electrons in their valence shell. A potassium atom loses 1 electron to for a K ion single and a sulphur atom gains 2 to form S2-. What is the charge of a potassium ion with 19 protons and 18 electrons.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many electrons does potassium lose by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






