Your How many electrons does potassium have per shell images are ready in this website. How many electrons does potassium have per shell are a topic that is being searched for and liked by netizens now. You can Find and Download the How many electrons does potassium have per shell files here. Download all free vectors.
If you’re looking for how many electrons does potassium have per shell images information linked to the how many electrons does potassium have per shell interest, you have come to the ideal site. Our site frequently provides you with hints for downloading the highest quality video and picture content, please kindly search and locate more enlightening video content and images that fit your interests.
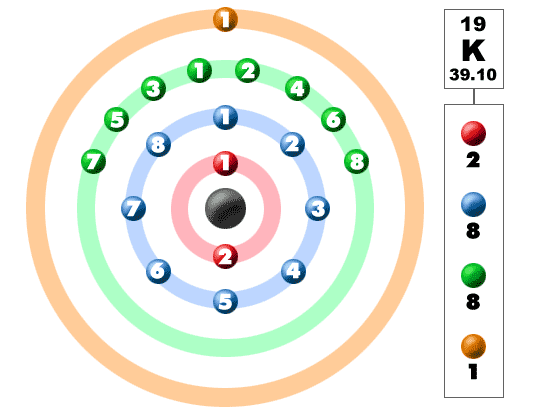
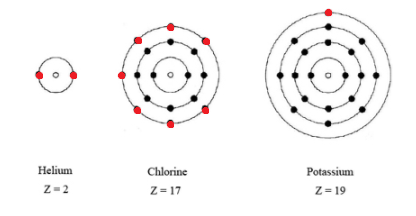
How Many Electrons Does Potassium Have Per Shell. How many electron shells does potassium K have. 04092009 201132 by chris. 086 grams per centimeter cubedMelting Point. That is the first shell of potassiumK has two electrons the second shell has eight electrons the 3rd shell has eight electrons and the 4 th shelllast orbit has one electron.
 Chem4kids Com Potassium Orbital And Bonding Info From chem4kids.com
Chem4kids Com Potassium Orbital And Bonding Info From chem4kids.com
Most elements that are essential in chemistry require eight electrons in each shell to be stable. And so for potassium there is ONE VALENCE electron that sits atop an ARGON core of. If we just go by the 2n2 model potassium ought to have nine valence electrons. Sir Humphry Davy in 1807 Potassium is the fourth element in the first column of the. Find more answers Ask your question New questions in Chemistry. How many valence electrons does potassium have.
The outer shell ofpotassium contains one electron.
You already know about the the first row. HOW MANY ELECTRONS DOES POTASSIUM HAVE. Potassium has an atomic number of. The first two shells completely fill up and the third fills up halfway. Sir Humphry Davy in 1807 Potassium is the fourth element in the first column of the. The second can hold up to eight electrons the third to 18 and so on.
 Source: biologydictionary.net
Source: biologydictionary.net
Valency of Potassium K. Why does potassium have 9 electrons in 3rd shell. The second can hold up to eight electrons the third to 18 and so on. An atom that has the maximum number of electrons in its outer shell. Most elements that are essential in chemistry require eight electrons in each shell to be stable.
 Source: tulane.edu
Source: tulane.edu
But that isnt possible. How many valence electrons does potassium have. How many electrons are in the outer energy levels of the element potassium. Potassium has an atomic number of. The general formula is that the nth shell can in principle hold up to 2 n 2 electrons.
 Source: br.pinterest.com
Source: br.pinterest.com
Like other elements in the first row potassium is a member of the alkali group with sodium and cesium. Potassium has an atomic number of. Traces of 40K are found in all potassium and it. How many electrons are in each shell. The outer shell ofpotassium contains one electron.
 Source: periodictableguide.com
Source: periodictableguide.com
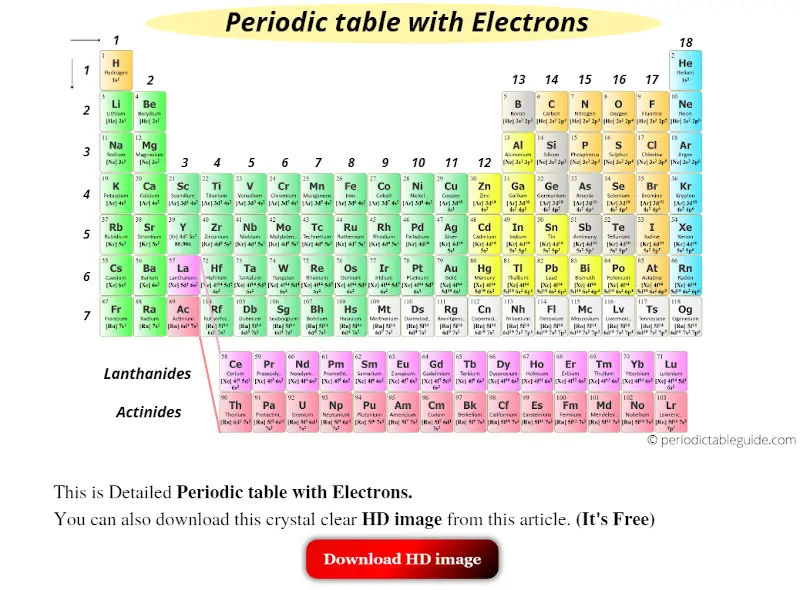
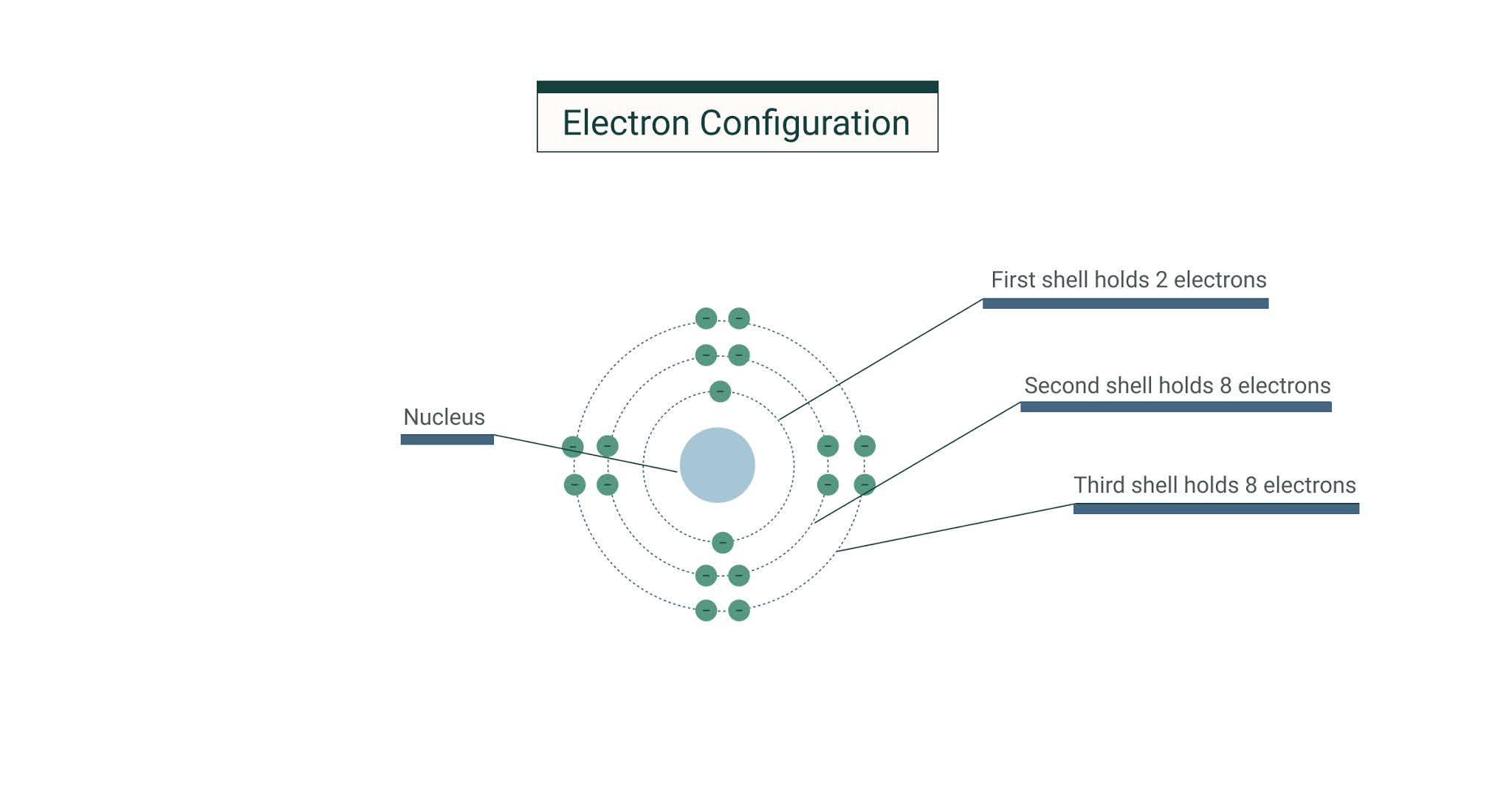
The first shell can hold up to two electrons the second shell can hold up to eight 2 6 electrons the third shell can hold up to 18 2 6 10 and so on. Of Arts and Science Philosophy Chemistry University of Windsor 2016 Answered 4 years ago. And potassium lies in Group 1 of the Periodic Table ie. Alkali metalPhase at Room Temperature. Of electronsshell 2 8 8 1 Group 1 Does potassium have 4 shells.
 Source: sciencenotes.org
Source: sciencenotes.org
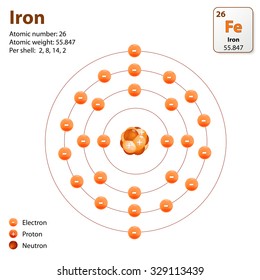
We know that the atom number of potassium is 19So potassium has 19 protons and 19 electrons together the charge of electrons and also protons are equal but opposite in natureThe fee of proton is 1 and also the charge of electron is -1. It is an alkali metal. However some atoms can be stable with. Valency of Potassium K. We know that the atom number of potassium is 19So potassium has 19 protons and 19 electrons together the charge of electrons and also protons are equal but opposite in natureThe fee of proton is 1 and also the charge of electron is -1.
 Source: pinterest.com
Source: pinterest.com
It can attain a stableconfiguration by loosing this electron. Each shell consists of one or more subshells and each subshell consists of one or more atomic orbitalsList of elements with electrons per shell. Oxygen chlorine potassium 6. Yes each shell can contain only fixed electron shell numbers. Each shell can only hold a certain amount of electrons and each shell must be filled before you move on to the next one.
 Source: chem.libretexts.org
Source: chem.libretexts.org
The outer shell ofpotassium contains one electron. All of the alkali metals have a single valence electron in the outer electron shell which is easily removed to create an ion with a positive charge a cation which combines with anions to form salts. But that isnt possible. How many electrons are in each shell. Of course the Periodic table.
 Source: pinterest.com
Source: pinterest.com
And the Group number specifies the number of valence electrons ie. However some atoms can be stable with. You already know about the the first row. Each shell can only hold a certain amount of electrons and each shell must be filled before you move on to the next one. Placing the electrons on the shells.
 Source: chem4kids.com
Source: chem4kids.com
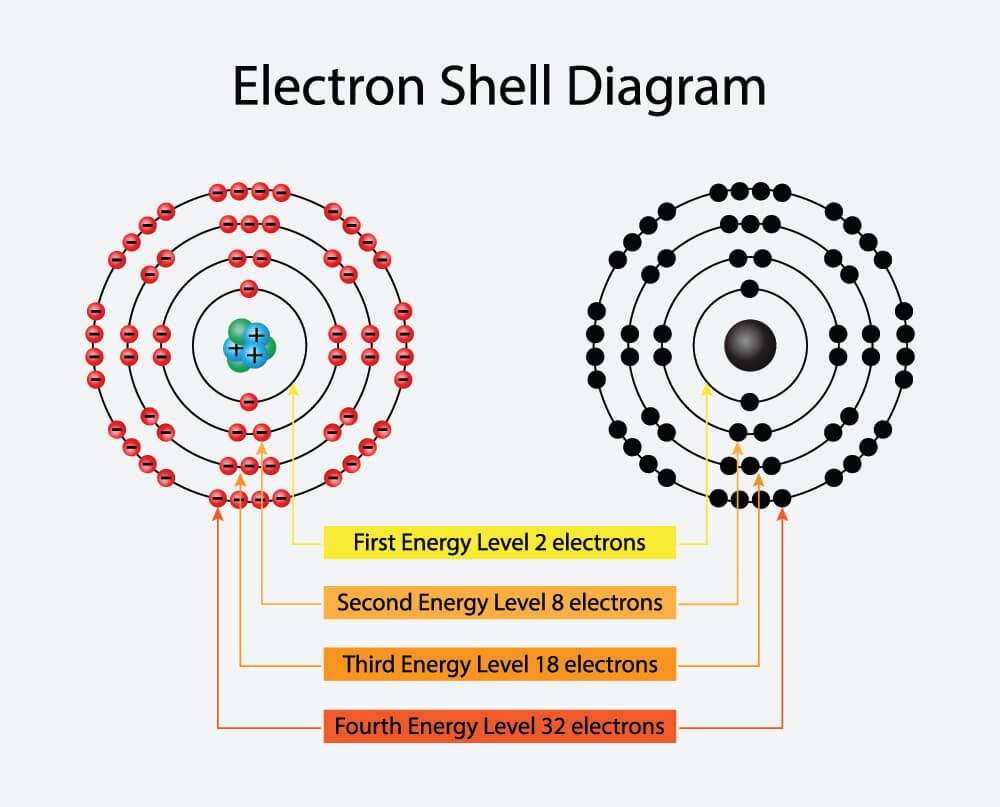
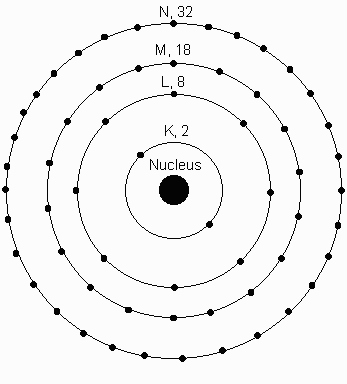
The first shell can hold 2 electrons. How many electron shells does potassium K have. The number of electrons in each shell in atomic structure is N e 2n 2 So the 3rd shell can have a maximum number of electrons of 18. The maximum number of electrons in each shell going from the middle to the outside is 2 8 8 18. The general formula is that the nth shell can in principle hold up to 2 n 2 electrons.
 Source: technologyuk.net
Source: technologyuk.net
The first shell can hold up to two electrons second up to eight electrons third up to 18 electrons and the subsequent shells can have up to 32 electrons in each. HOW MANY ELECTRONS DOES POTASSIUM HAVE. Potassium is one of the alkali metals. But that isnt possible. The first two shells completely fill up and the third fills up halfway.
 Source: sciencenotes.org
Source: sciencenotes.org
Find more answers Ask your question New questions in Chemistry. Each shell can contain only a fixed number of electrons. Alkali metalPhase in Room Temperature. Thus potassium has only one valence electron. The first shell can hold up to two electrons second up to eight electrons third up to 18 electrons and the subsequent shells can have up to 32 electrons in each.

The second can hold up to eight electrons the third to 18 and so on. How many electrons does potassium have. Thus potassium has only one valence electron. Because there is only one electron the element is very reactive and searches out other elements to make new compounds. It can attain a stableconfiguration by loosing this electron.
 Source: valenceelectrons.com
Source: valenceelectrons.com
The 3rd shell of any atom can actually contain 18 electrons but potassium K normally has only one electron in the 4s orbital. 2nd 8 electrons. Each shell can contain only a fixed number of electrons. The first shell can hold 2 electrons. It can attain a stableconfiguration by loosing this electron.
 Source: shutterstock.com
Source: shutterstock.com
The number of electrons in the valence shell. Of course the Periodic table. We know that the atom number of potassium is 19So potassium has 19 protons and 19 electrons together the charge of electrons and also protons are equal but opposite in natureThe fee of proton is 1 and also the charge of electron is -1. How many electrons are in the outer energy levels of the element potassium. The general formula is that the nth shell can in principle hold up to 2 n 2 electrons.
 Source: kanayatichemistry.blogspot.com
Source: kanayatichemistry.blogspot.com
An atom that has the maximum number of electrons in its outer shell. Potassium is one of the alkali metals. HOW MANY ELECTRONS DOES POTASSIUM HAVE. The 3rd shell of any atom can actually contain 18 electrons but potassium K normally has only one electron in the 4s orbital. Alkali metalPhase in Room Temperature.
 Source: valenceelectrons.com
Source: valenceelectrons.com
The first two shells completely fill up and the third fills up halfway. How many valence electrons does potassium have. Thus potassium has only one valence electron. The general formula is that the nth shell can in principle hold up to 2 n 2 electrons. How many electron shells does potassium K have.

Alkali metalPhase at Room Temperature. And potassium lies in Group 1 of the Periodic Table ie. Each shell can contain only a fixed number of electrons. The electron configuration of potassiumK through the sub-orbit is 1s. The first shell can hold 2 electrons.
 Source: shutterstock.com
Source: shutterstock.com
Alkali metalPhase at Room Temperature. To put an electron into a 3d orbital you need to reach a -2 charge but its far more energetic. Sir Humphry Davy in 1807 Potassium is the fourth aspect in the very first. All of the alkali metals have a single valence electron in the outer electron shell which is easily removed to create an ion with a positive charge a cation which combines with anions to form salts. All of the members of the alkali group have an outer shell with only one electron in orbit.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many electrons does potassium have per shell by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






