Your Elements of the periodic table are distinguished by images are ready in this website. Elements of the periodic table are distinguished by are a topic that is being searched for and liked by netizens now. You can Download the Elements of the periodic table are distinguished by files here. Get all royalty-free photos and vectors.
If you’re looking for elements of the periodic table are distinguished by images information related to the elements of the periodic table are distinguished by topic, you have visit the ideal blog. Our website frequently provides you with hints for refferencing the maximum quality video and picture content, please kindly surf and locate more enlightening video articles and graphics that match your interests.
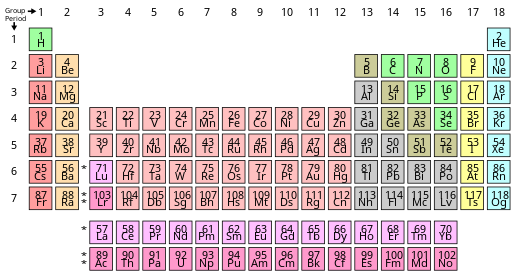
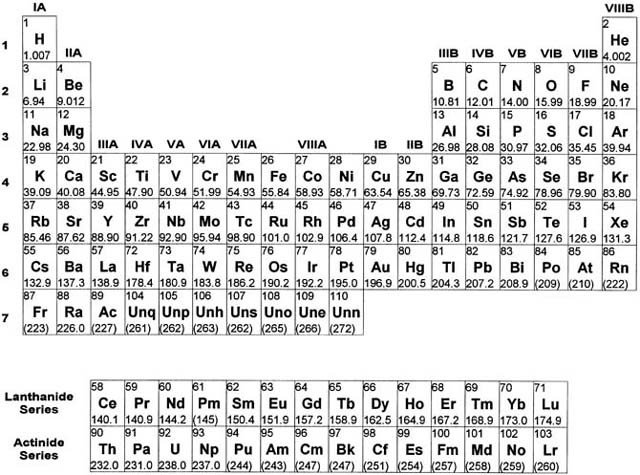
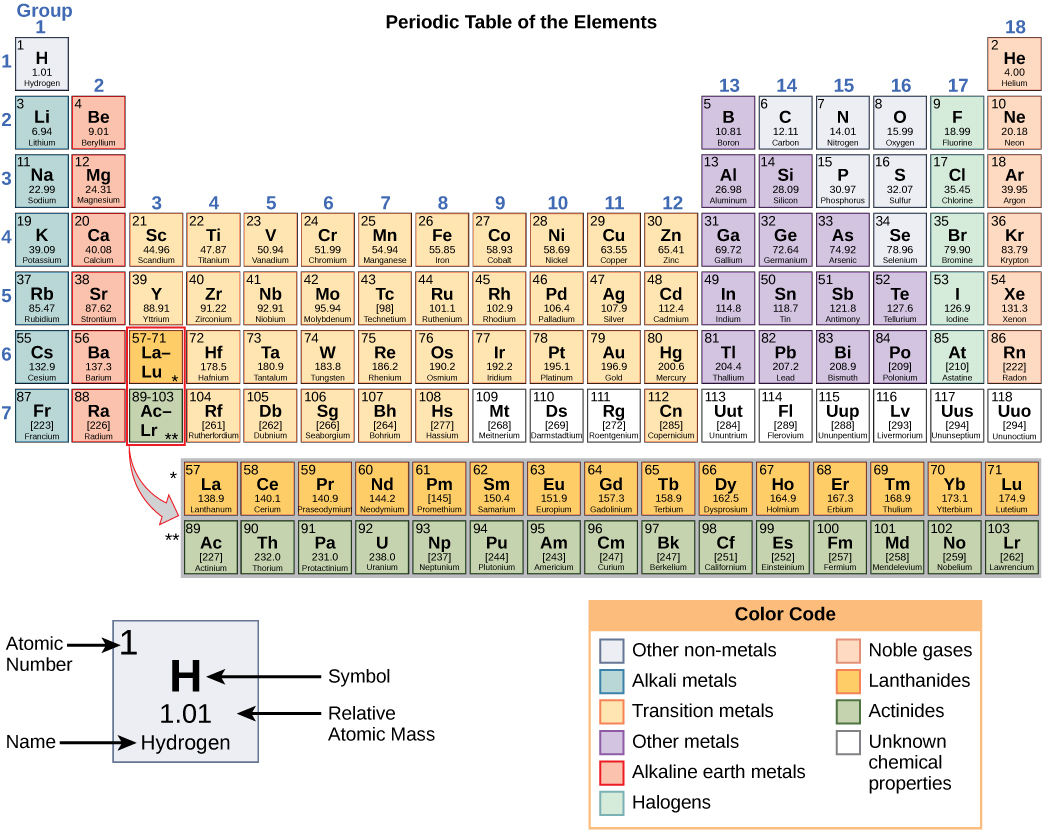
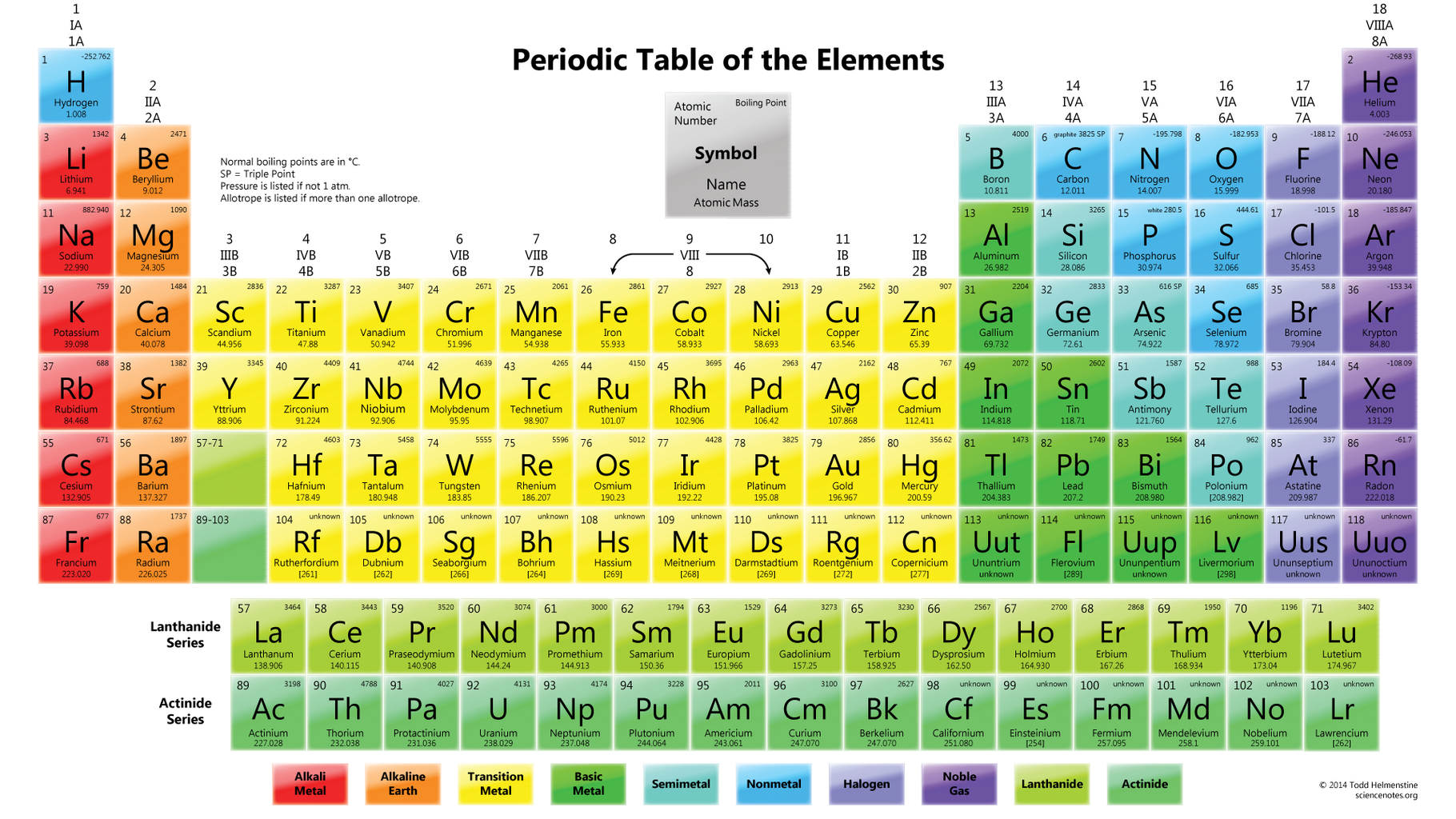
Elements Of The Periodic Table Are Distinguished By. The mass number is always a whole number and has units of nucleons. Each atom type contains the same number of protons. There are 92 naturally-occurring elements on earth. The periods and group are distinguished properly.
 Elements And The Periodic Table Introductory Chemistry Lecture Lab From courses.lumenlearning.com
Elements And The Periodic Table Introductory Chemistry Lecture Lab From courses.lumenlearning.com
N - Boiling point. The elements in groups 1014 except boron and carbon have very complex AgM phase. Different isotopes of a given element are distinguished by their mass numbers which are conventionally written as a superscript on the left hand side of the atomic symbol eg. Start studying Chemistry Study Questions - Elements Compounds Mixtures and The Periodic Table. The periodic table organizes elements according to INCREASING Atomic number. The elements among the options are.
Different isotopes of a given element are distinguished by their mass numbers which are conventionally written as a superscript on the left hand side of the atomic symbol eg.
Ca Fe Si and Ne. A chemical element is a pure chemical substance consisting of a single type of atom distinguished by its atomic number which is the number of protons in its atomic nucleus. In the Periodic Table elements are arranged according to their atomic number and they are grouped according to similar chemical properties and are depicted by their symbols. B The number of neutrons in the nucleus. What distinguishes one isotope. Periodic table Common examples of elements also shown in peridic table are hydrogen carbon nitrogen and oxygen.
 Source: wikiwand.com
Source: wikiwand.com
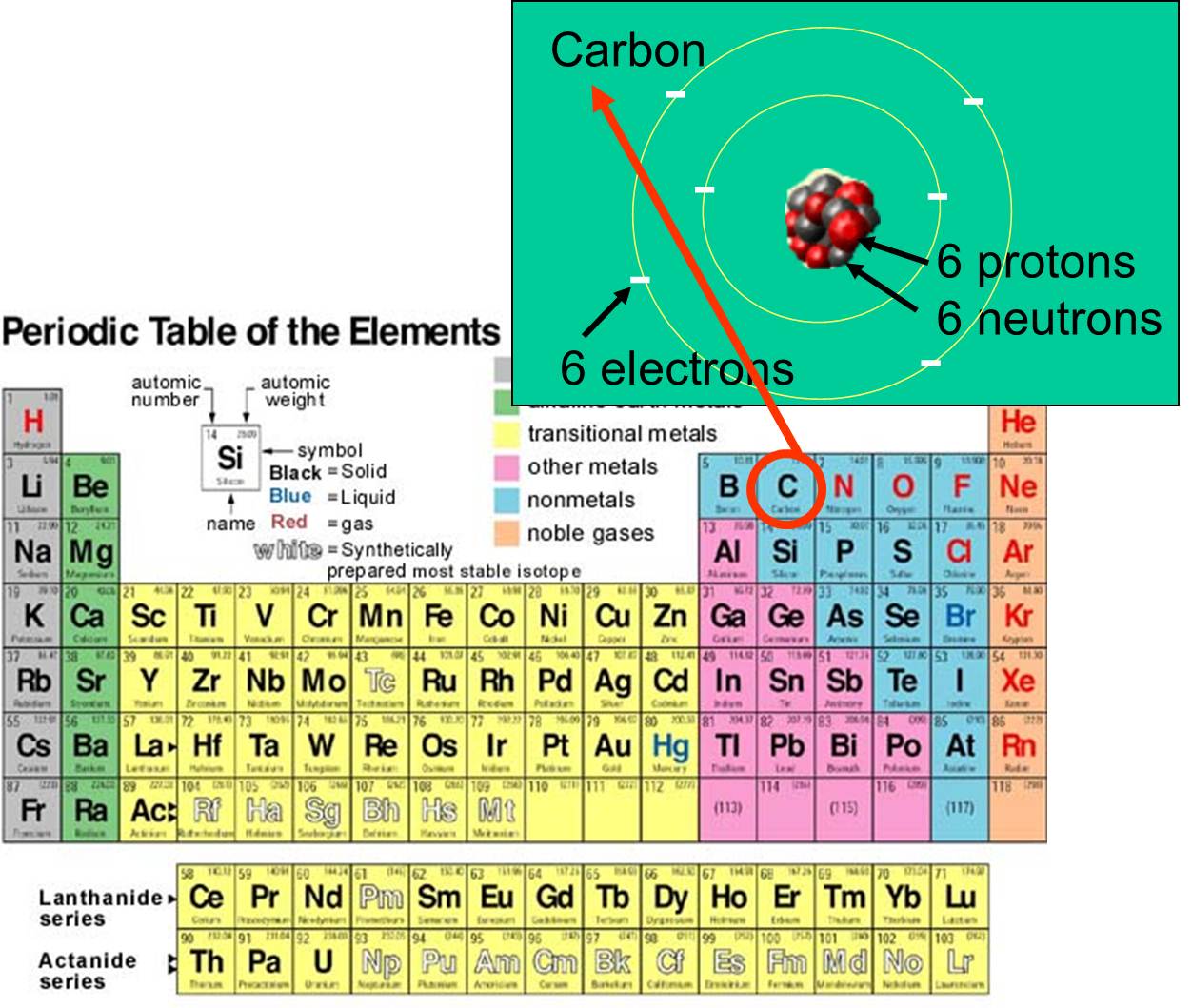
Each element is distinguished by its atomic number ie the number of protons in the nuclei of its atoms. An element is a material that consists of a single type of atom. The 6 charged electrons contribute very little to the atomic mass. Periodic table Common examples of elements also shown in peridic table are hydrogen carbon nitrogen and oxygen. How can elements be distinguished.
 Source: pinterest.com
Source: pinterest.com
At 150 years old the table is still growing. Their atomic numbers the number of protons in the nucleus that determines their chemical properties and place in the periodic table are 113 115 117 and 118 respectively. The periodic table contains a variety of metallic elements which include alkali metals alkali earth metals transition metals. In the Periodic Table elements are arranged according to their atomic number and they are grouped according to similar chemical properties and are depicted by their symbols. No new chemical bonds are formed.
 Source: pinterest.com
Source: pinterest.com
Each atom type contains the same number of protons. Elements of the Periodic Table are distinguished by a The number of protons in the nucleus. A chemical element is a pure chemical substance consisting of a single type of atom distinguished by its atomic number which is the number of protons in its atomic nucleus. Learn vocabulary terms and more with flashcards games and other study tools. The periodic table of the chemical elements A chemical element is a type of atom that is distinguished by its atomic number.
 Source: researchgate.net
Source: researchgate.net
The vector stencils library Periodic table of chemical elements contains 119 icon symbols of chemical elements for drawing Mendeleevs periodic table chemical diagrams infographics and illustrations. There is a recurring pattern called the periodic law in their properties in which elements in the same column group have similar properties. Li Na K Rb Cs and Fr. Each element is distinguished by the number of protons neutrons and electrons that it possess. C - Melting point.
 Source: pinterest.com
Source: pinterest.com
The elements from groups 13 except for hydrogen lithium and beryllium are very miscible with silver in the condensed phase and form intermetallic compounds. A nucleus has a More energy than. The creator of the periodic table Dmitri Mendeleev in 1869 began collecting and sorting known properties of elements like he was playing a game while traveling by train. The 6 charged electrons contribute very little to the atomic mass. The periodic table contains a variety of metallic elements which include alkali metals alkali earth metals transition metals.
 Source: csun.edu
Source: csun.edu
B The number of neutrons in the nucleus. Each element is distinguished by its atomic number ie the number of protons in the nuclei of its atoms. Their atomic numbers the number of protons in the nucleus that determines their chemical properties and place in the periodic table are 113 115 117 and 118 respectively. The modern periodic table is based on the modern periodic law put forward by the English physicist Henry Moseley which states that. Also if you want to know the chemical element name simply hover over it.
 Source: link.springer.com
Source: link.springer.com
The periodic table is the tabular arrangement of all the chemical elements on the basis of their respective atomic numbers. O - Vanderwaals radius. Li - Atomic Mass. The chemical elements of the periodic chart sorted by. The periods and group are distinguished properly.
 Source: sphweb.bumc.bu.edu
Source: sphweb.bumc.bu.edu
Element Symbol The elements of the periodic table sorted by symbol The chemical elements of the periodic chart sorted by. F - Year of. Different isotopes of a given element are distinguished by their mass numbers which are conventionally written as a superscript on the left hand side of the atomic symbol eg. Each element corresponds to a single entry on the periodic table. Be Mg Ca Sr Ba and Ra.
 Source: fi.pinterest.com
Source: fi.pinterest.com
The creator of the periodic table Dmitri Mendeleev in 1869 began collecting and sorting known properties of elements like he was playing a game while traveling by train. Also if you want to know the chemical element name simply hover over it. N P As Sb Bi and. D Both a and b. What is mixture of elements.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
F - Year of. Symbol - Name alphabetically. Elements of the Periodic Table are distinguished by a The number of protons in the nucleus. Start studying Chemistry Study Questions - Elements Compounds Mixtures and The Periodic Table. The creator of the periodic table Dmitri Mendeleev in 1869 began collecting and sorting known properties of elements like he was playing a game while traveling by train.
 Source: researchgate.net
Source: researchgate.net
F - Year of. The mass number is always a whole number and has units of nucleons. Their atomic numbers the number of protons in the nucleus that determines their chemical properties and place in the periodic table are 113 115 117 and 118 respectively. Different isotopes of a given element are distinguished by their mass numbers which are conventionally written as a superscript on the left hand side of the atomic symbol eg. Periodic table Common examples of elements also shown in peridic table are hydrogen carbon nitrogen and oxygen.
 Source: instructables.com
Source: instructables.com
Those from groups 49 are only poorly miscible. A mixture is made by simply mixing together elements and compounds. The first chemical element with the lowest density is Hydrogen and the highest density is Osmium. Symbol Name chemical element - Atomic number Ag Silver - Symbol Al Aluminum - Atomic Mass Am Americium. What is mixture of elements.
 Source: pinterest.com
Source: pinterest.com
The periodic table organizes elements according to INCREASING Atomic number. The vector stencils library Periodic table of chemical elements contains 119 icon symbols of chemical elements for drawing Mendeleevs periodic table chemical diagrams infographics and illustrations. N - Boiling point. Also if you want to know the chemical element name simply hover over it. For example carbons atomic number is 6 and has an atomic mass of about 12 because it has 6 positively charged protons and 6 non-charged neutrons.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Element Symbol The elements of the periodic table sorted by symbol The chemical elements of the periodic chart sorted by. The creator of the periodic table Dmitri Mendeleev in 1869 began collecting and sorting known properties of elements like he was playing a game while traveling by train. Each element is distinguished by its atomic number ie the number of protons in the nuclei of its atoms. Seeing chemical elements arranged in the modern periodic table is as familiar as seeing a map of the world but it was not always so obvious. The periodic table is a tabular arrangement of the chemical elements.

On hover the name in a blue font appears in a pleasing way. Silver forms alloys with most other elements on the periodic table. The vector stencils library Periodic table of chemical elements contains 119 icon symbols of chemical elements for drawing Mendeleevs periodic table chemical diagrams infographics and illustrations. E a b and c. It took a decade and worldwide effort to confirm these.
 Source: pinterest.com
Source: pinterest.com
Li - Atomic Mass. Please note that the elements do not show their natural relation towards each other as in the Periodic system. Different isotopes of a given element are distinguished by their mass numbers which are conventionally written as a superscript on the left hand side of the atomic symbol eg. Elements are distinguished by their name symbol atomic number melting point boiling point density and ionization energies. N - Boiling point.

Start studying Chemistry Study Questions - Elements Compounds Mixtures and The Periodic Table. There is a recurring pattern called the periodic law in their properties in which elements in the same column group have similar properties. The first chemical element with the lowest density is Hydrogen and the highest density is Osmium. Sc Y La Ce Pr Nd Pm Sm Eu Gd Tb Tb Dy Ho Er Tm Yb and Lu. The periodic table of the chemical elements A chemical element is a type of atom that is distinguished by its atomic number.
 Source: pinterest.com
Source: pinterest.com
The mass number is always a whole number and has units of nucleons. N P As Sb Bi and. No new chemical bonds are formed. Seeing chemical elements arranged in the modern periodic table is as familiar as seeing a map of the world but it was not always so obvious. Ca Fe Si and Ne.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title elements of the periodic table are distinguished by by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






