Your Chemistry periodic table regents images are ready. Chemistry periodic table regents are a topic that is being searched for and liked by netizens now. You can Find and Download the Chemistry periodic table regents files here. Find and Download all royalty-free photos and vectors.
If you’re looking for chemistry periodic table regents pictures information related to the chemistry periodic table regents topic, you have come to the right blog. Our website frequently provides you with hints for seeing the highest quality video and picture content, please kindly surf and locate more enlightening video content and images that match your interests.
Chemistry Periodic Table Regents. The Chemisty Regents Exam is broken down into three sections. Electronegativity values for an element are provided in Table S. Course of the school year. Trends in the Periodic Table Homework Unit 7 - Topic 4 Regents What are the trends in the Periodic Table.
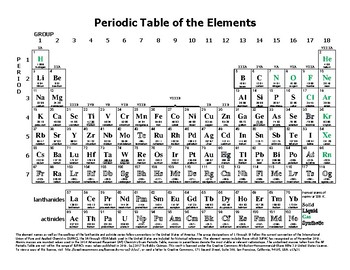 Periodic Table Of Elements Nys Regents Periodic Table Timeline From utedzz.blogspot.com
Periodic Table Of Elements Nys Regents Periodic Table Timeline From utedzz.blogspot.com
The booklet is frequently used during classes tests and lab assignments. Unit 4- The Periodic Table - MS. Below is a portion of the periodic table. An element in Group 1 of the periodic table that is extremely reactive. The content is divided into two parts - Alkaline earth metal and another video has alre. Unit Objectives use for Evidence of Study.
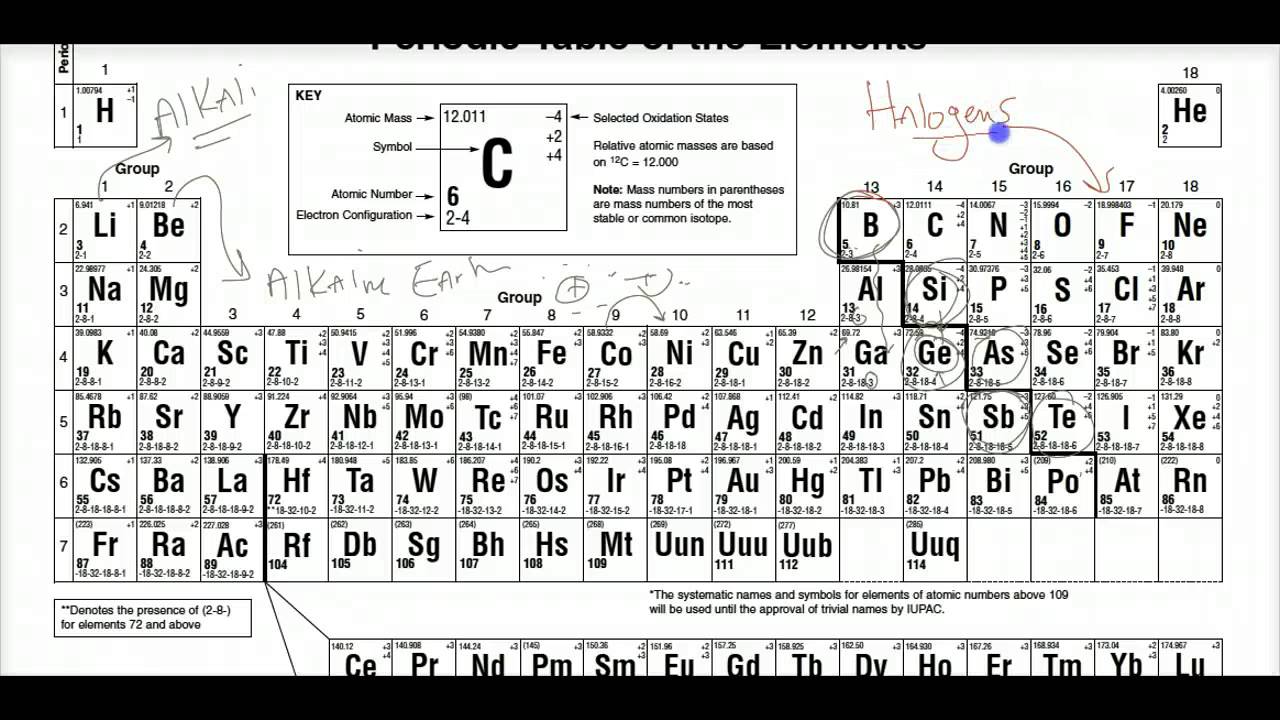
18 As we move down Group 1 elements of the Periodic Table the first ionization energy of each element decreases.
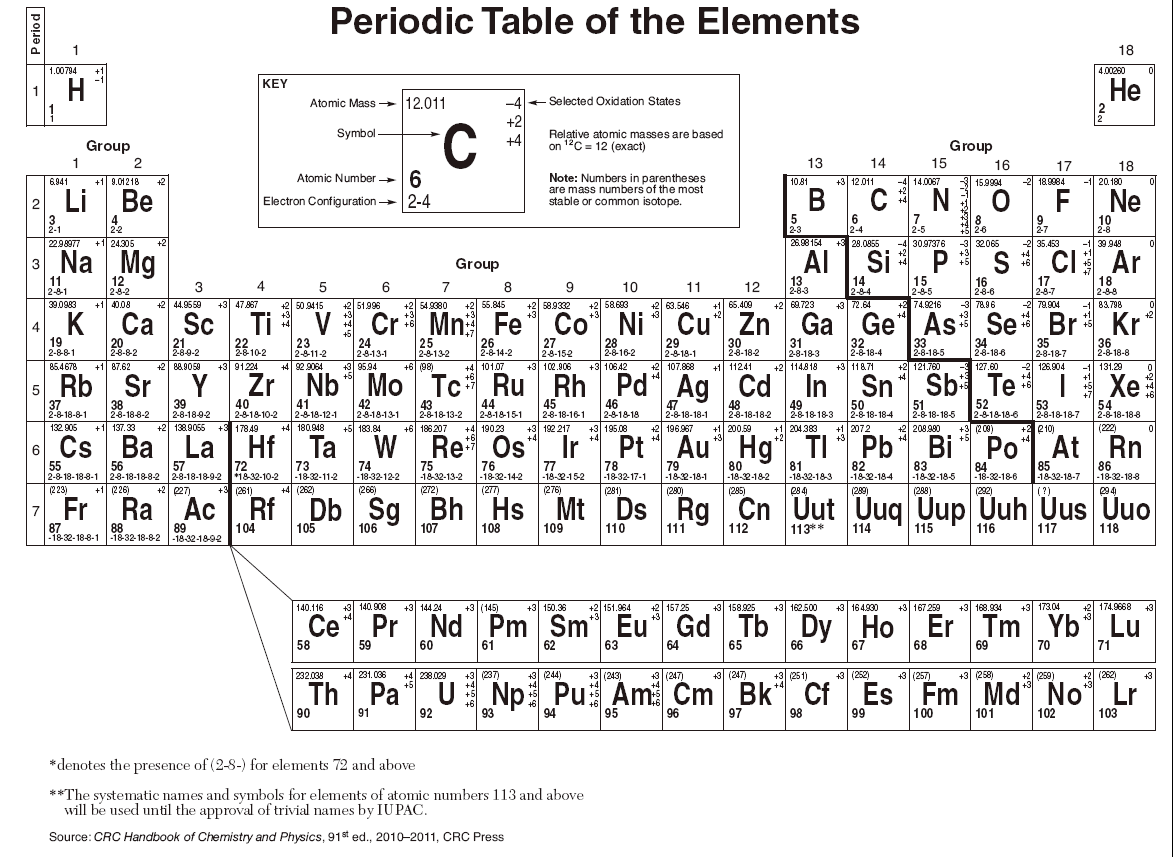
The size of an atom. The Chemistry reference table CRT is a type of booklet that contains important information such as measurement equations charts formulas standard values guidelines for various properties identification tables etc related to the chemistry core concept. The key tables and charts included in the Chemistry reference table-. Learn vocabulary terms and more with flashcards games and other study tools. 18 As we move down Group 1 elements of the Periodic Table the first ionization energy of each element decreases. It contains important measurements equations and identification tables.
 Source: angelfire.com
Source: angelfire.com
This quiz is incomplete. The diagram below represents the nucleus of an atom. Then answer the questions that follow. This quiz is incomplete. REGENTS CHEMISTRY Name_____ Period_____ Chemistry Review WebQuest Directions.
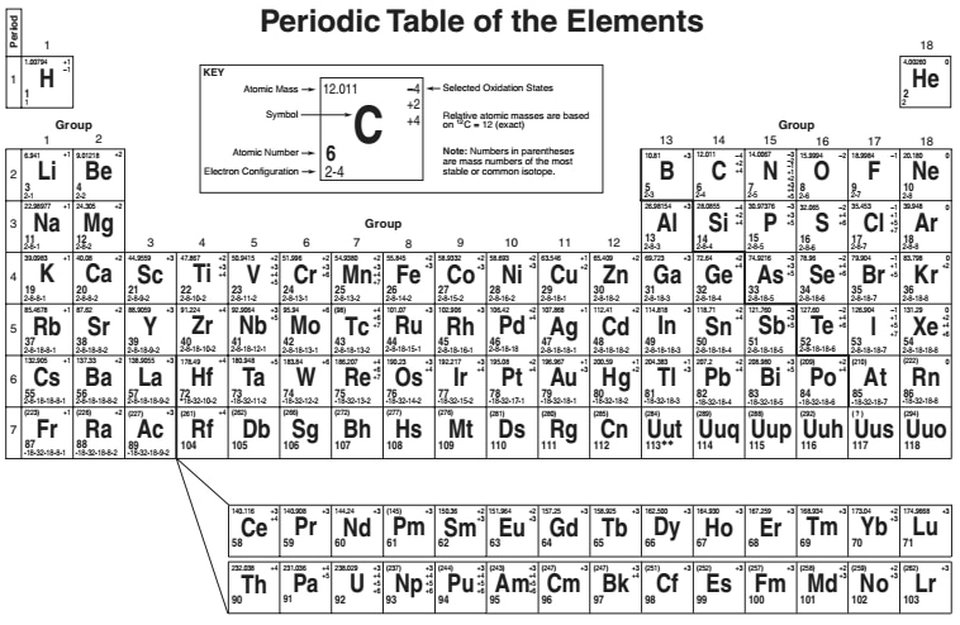 Source: forwardthinkingmuseum.org
Source: forwardthinkingmuseum.org
The Chemistry reference table CRT is a type of booklet that contains important information such as measurement equations charts formulas standard values guidelines for various properties identification tables etc related to the chemistry core concept. Host a game. The diagram below represents the nucleus of an atom. _____ 10 pts 2. A high ionization energy and good electrical conductivity B low ionization energy and good electrical conductivity C high ionization energy and poor electrical conductivity D low ionization energy and poor electrical conductivity.
 Source: utedzz.blogspot.com
Source: utedzz.blogspot.com
Mass numbers in parentheses are mass numbers of. The key tables and charts included in the Chemistry reference table-. An element in Group 2 of the periodic table that is very reactive. The Periodic Table is made up of PERIODS and GROUPS. 44 Easy Free Download chemistry periodic table regents Now.
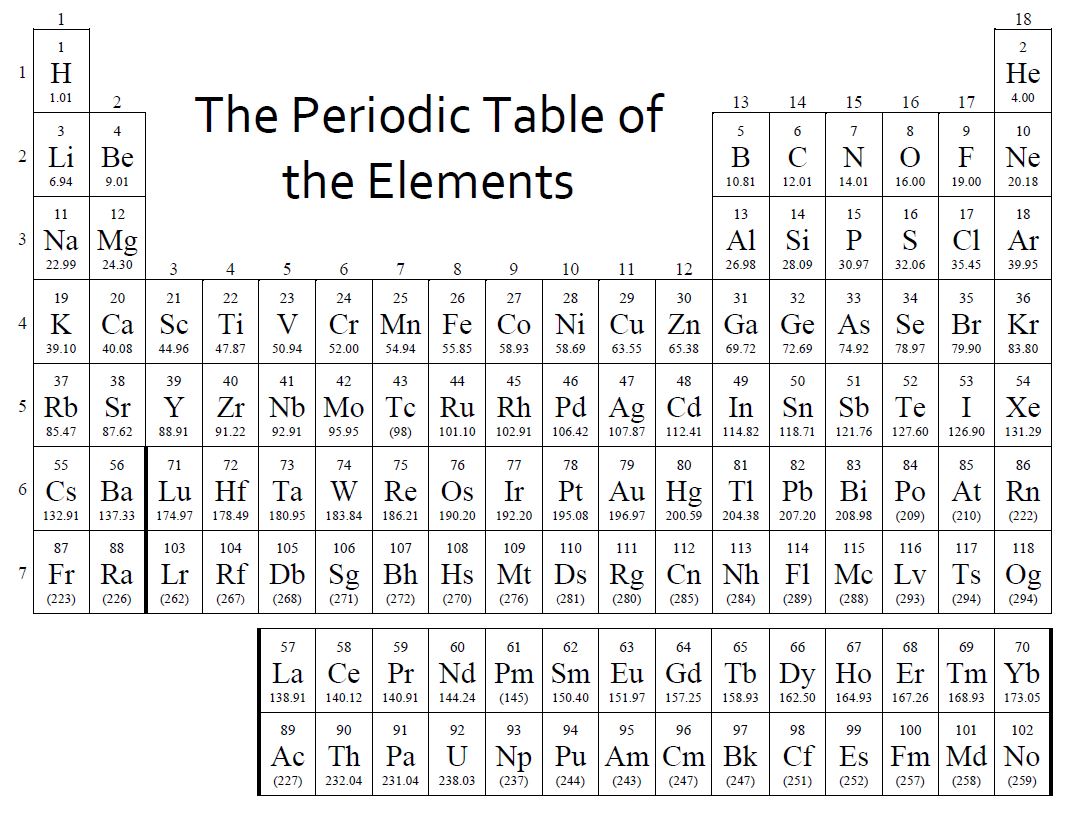 Source: utedzz.blogspot.com
Source: utedzz.blogspot.com
D The atomic number is 11 and the mass number is 20. The booklet is frequently used during classes tests and lab assignments. To play this quiz please finish editing it. The Periodic Table is made up of PERIODS and GROUPS. BThe atomic number is 9 and the mass number is 20.
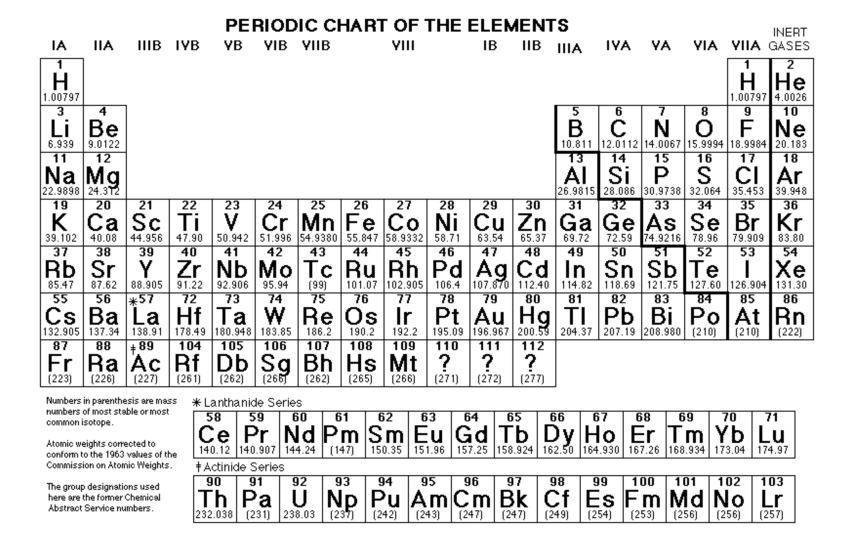 Source: utedzz.blogspot.com
Source: utedzz.blogspot.com
Regents Chemistry Periodic Table Review Part 1 Basics of the Periodic Table. Learn vocabulary terms and more with flashcards games and other study tools. The Chemistry reference table CRT is a type of booklet that contains important information such as measurement equations charts formulas standard values guidelines for various properties identification tables etc related to the chemistry core concept. Tables graphing and laboratory experiments. It contains important measurements equations and identification tables.
 Source: bash-le-1.weebly.com
Source: bash-le-1.weebly.com
Follow the instructions below and fill in the blanks to receive credit. Arrangement of the Periodic Table. Found on the Periodic Table Cannot be broken down physically or chemically Can be chemically separated Fixed ratio of components Properties of compound are different than individual components Written with formulas Not pure substances Physically combined can be physically separated Variable ratio Individual components retain properties. 26State in terms of the number of electron shells why the radius of a strontium atom in the ground state is larger than the radius of. An element in Group 2 of the periodic table that is very reactive.
 Source: cl.castlelearning.com
Source: cl.castlelearning.com
You can compare the electronegativities of any two elements to determine which one will attract electrons more. Before you start have out your reference table. This video explains about s- Block elements of modern periodic table. If the electronegativity is the. Regents Review for Atomic Structure DRAFT.
 Source: utedzz.blogspot.com
Source: utedzz.blogspot.com
The CRT is also used on the Chemistry Regents Exam. The CRT is also used on the Chemistry Regents Exam. Learn vocabulary terms and more with flashcards games and other study tools. The content is divided into two parts - Alkaline earth metal and another video has alre. Trends in the Periodic Table Homework Unit 7 - Topic 4 Regents What are the trends in the Periodic Table.
 Source: utedzz.blogspot.com
Source: utedzz.blogspot.com
The content is divided into two parts - Alkaline earth metal and another video has alre. 26State in terms of the number of electron shells why the radius of a strontium atom in the ground state is larger than the radius of. 18 As we move down Group 1 elements of the Periodic Table the first ionization energy of each element decreases. One reason for this is that a the nuclear charge is decreasing b the number of principal energy levels is decreasing c the distance between the valence electron and the nucleus is increasing d the number of neutrons is increasing. To play this quiz please finish editing it.

Then answer the questions that follow. PERIODS HORIZONTAL ROWS run left to right OCTET full VALENCE SHELL 8 electrons except for PERIOD 1 elements for whom 2e-marks a full valence shell RememberEIGHT IS GREAT. The Chemistry reference table CRT is a type of booklet that contains important information such as measurement equations charts formulas standard values guidelines for various properties identification tables etc related to the chemistry core concept. This tutorial breaks down the properties of each group and examines concepts such as chemical activity electronegativity ionization energy and number of va. For example oxygen has a greater attraction for an electron than a metal like lithium because it has a higher electronegativity value see Table S.
 Source: quizlet.com
Source: quizlet.com
One reason for this is that a the nuclear charge is decreasing b the number of principal energy levels is decreasing c the distance between the valence electron and the nucleus is increasing d the number of neutrons is increasing. Click on the Notes packet. Questions focus on the Reference. In the answer spaces provided fill in 1 atomic number 2 atomic radius 3 number of shells and 4 number of valence electrons. REGENTS CHEMISTRY Name_____ Period_____ Chemistry Review WebQuest Directions.

A high ionization energy and good electrical conductivity B low ionization energy and good electrical conductivity C high ionization energy and poor electrical conductivity D low ionization energy and poor electrical conductivity. Unit Objectives use for Evidence of Study. REGENTS CHEMISTRY Name_____ Period_____ Chemistry Review WebQuest Directions. Course of the school year. The Chemisty Regents Exam is broken down into three sections.
 Source: youtube.com
Source: youtube.com
26State in terms of the number of electron shells why the radius of a strontium atom in the ground state is larger than the radius of. 26State in terms of the number of electron shells why the radius of a strontium atom in the ground state is larger than the radius of. 10th - 11th grade. Follow the instructions below and fill in the blanks to receive credit. Regents Review for Atomic Structure DRAFT.
 Source: utedzz.blogspot.com
Source: utedzz.blogspot.com
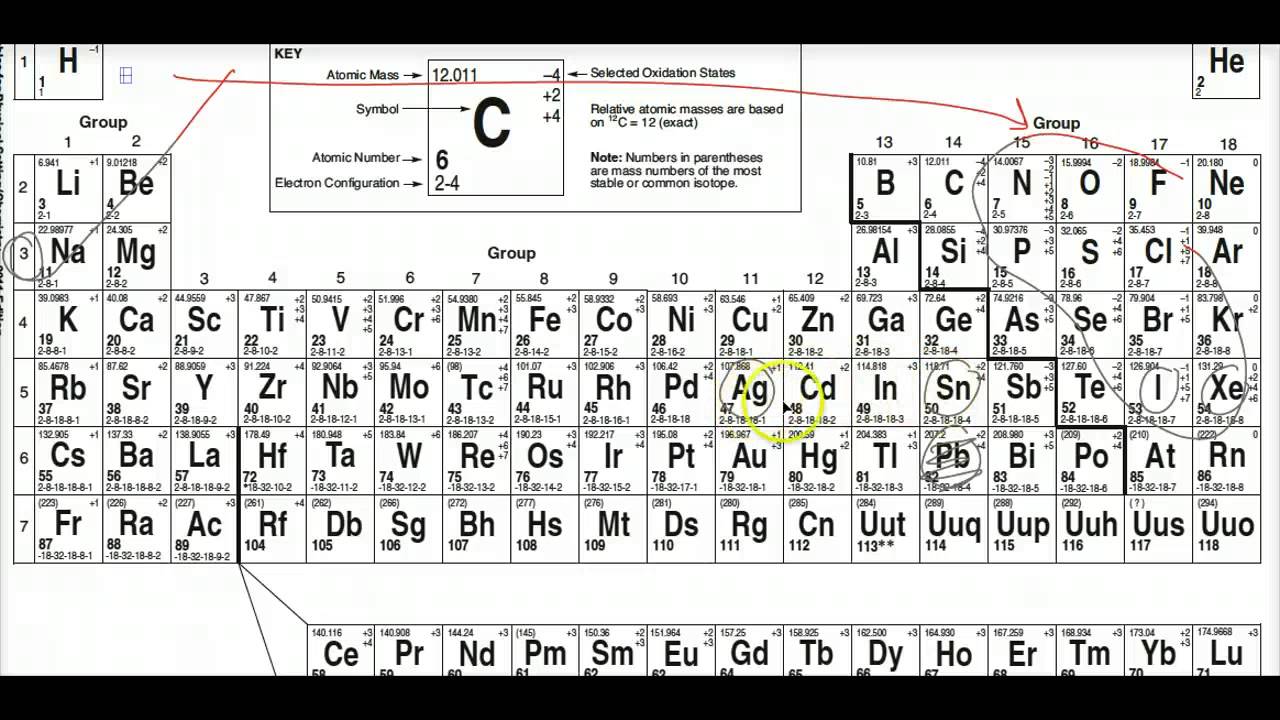
Learn vocabulary terms and more with flashcards games and other study tools. Follow the instructions below and fill in the blanks to receive credit. D The atomic number is 11 and the mass number is 20. Periodic Table of the Elements C 12011 4 2 4 6 2-4 Atomic Mass Symbol Atomic Number Electron Configuration Selected Oxidation States Relative atomic masses are based on 12 C 12000 Note. The diagram below represents the nucleus of an atom.
 Source: chemistry.ucla.edu
Source: chemistry.ucla.edu
Learn vocabulary terms and more with flashcards games and other study tools. 18 As we move down Group 1 elements of the Periodic Table the first ionization energy of each element decreases. Click on Unit 5. Mass numbers in parentheses are mass numbers of. Murdoch Website upload 2014 Page 3 of 49 Unit 6 Vocabulary.
 Source: youtube.com
Source: youtube.com
The Period Table Bonding-key Regents Chemistry 14- Z15 Mr. Chemistry Reference Tables The Chemistry Reference Tables CRT is an invaluable tool to the chemistry student. This tutorial breaks down the properties of each group and examines concepts such as chemical activity electronegativity ionization energy and number of va. Regents Review for Atomic Structure DRAFT. Follow the instructions below and fill in the blanks to receive credit.

_____ 10 pts 2. Element Symbol Element Name Group Electron configuration Lewis diagram. The key tables and charts included in the Chemistry reference table-. Found on the Periodic Table Cannot be broken down physically or chemically Can be chemically separated Fixed ratio of components Properties of compound are different than individual components Written with formulas Not pure substances Physically combined can be physically separated Variable ratio Individual components retain properties. Mass numbers in parentheses are mass numbers of.
 Source: sites.google.com
Source: sites.google.com
A high ionization energy and good electrical conductivity B low ionization energy and good electrical conductivity C high ionization energy and poor electrical conductivity D low ionization energy and poor electrical conductivity. 26State in terms of the number of electron shells why the radius of a strontium atom in the ground state is larger than the radius of. 10th - 11th grade. Tables graphing and laboratory experiments. Trends in the Periodic Table Homework Unit 7 - Topic 4 Regents What are the trends in the Periodic Table.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title chemistry periodic table regents by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






