Your B element protons neutrons electrons images are ready in this website. B element protons neutrons electrons are a topic that is being searched for and liked by netizens today. You can Find and Download the B element protons neutrons electrons files here. Find and Download all free photos and vectors.
If you’re looking for b element protons neutrons electrons images information linked to the b element protons neutrons electrons keyword, you have pay a visit to the ideal site. Our website frequently gives you suggestions for seeking the highest quality video and picture content, please kindly search and find more informative video articles and graphics that match your interests.
B Element Protons Neutrons Electrons. The protons and neutrons are found in the nucleus of the atom. Rules to Finding Number of Protons Neutrons and Electrons of protons atomic number of neutrons mass number atomic number of electrons atomic number charge Thats it. The actual masses of protons electrons and neutrons are not known. Atomic number number of protons number of electrons.
 Boron Protons Neutrons Electrons Electron Configuration From material-properties.org
Boron Protons Neutrons Electrons Electron Configuration From material-properties.org
Protons carry a positive electrical change while electrons are negatively charged and neutrons are neutral. The number of neutrons depends on the isotope. 23000 C 257315 K 41720 F Boiling Point. The atomic or proton number Z of an atom is the number of protons in its nucleus. Atomic number number of protons number of electrons. Electrons have a negative charge.
The number of subatomic particles in atoms of different elements varies.
The actual mass of an electron is very large compared to the actual mass of a proton. Boron-10 is composed of 5 protons 5 neutrons and 5 electrons. An electron is usually. All oxide anions O2- have 8 protons and 10 electrons. C Y and Z are isotopes of elements in the. If the charge is positive there are more protons.

This means that it has gained two electrons from another element such as sodium or magnesium. 23000 C 257315 K 41720 F Boiling Point. Since you didnt specify which isotope Ill give the. The total number of protons in the atoms of an element is always equal to the atomic number of the element. An atom is composed of protons neutrons and electrons.
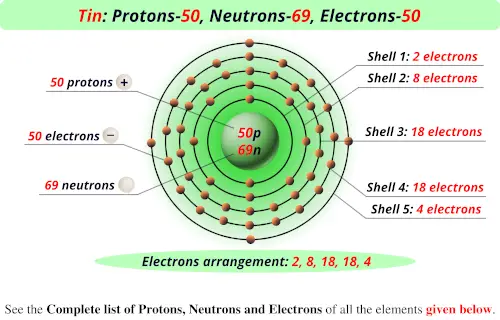 Source: periodictableguide.com
Source: periodictableguide.com
Neutrons have no charge. Protons have a positive charge. Its nalpha reaction cross-section for thermal. Hydrogen Z 1 has 1 proton and 1 electron while uranium with Z 92 has 92 protons and 92 electrons. Atomic number number of protons number of electrons.

Neutrons are electrically neutral particles and carry no charge. B X and Y are isotopes of elements in the same group of the Periodic Table. Its nalpha reaction cross-section for thermal. As summarized in Table 21 protons are positively charged neutrons are uncharged and electrons are negatively charged. It can be seen that valency of an element is related to number of valence electrons in that atom.
 Source: slideserve.com
Source: slideserve.com
Its nalpha reaction cross-section for thermal. A standard periodic table of elements can provide you with a great deal of insight into the composition of an atom. Controversial work has suggested that clusters of four neutrons might just be stable enough to call element zero an element with. Electrons have a negative charge. The atomic or proton number Z of an atom is the number of protons in its nucleus.
 Source: slideplayer.com
Source: slideplayer.com
B Mass or nucleon number A. Boron-10 is composed of 5 protons 5 neutrons and 5 electrons. C Y and Z are isotopes of elements in the. B Mass or nucleon number A. What are the Characteristics of Electron Proton and Neutron Electron Electron was discovered by JJ.
 Source: dreamstime.com
Source: dreamstime.com
23000 C 257315 K 41720 F Boiling Point. B X and Y are isotopes of elements in the same group of the Periodic Table. An atom is normally electrically neutral and so the atomic number is also the number of electrons in a neutral atom of the element. Neutrons are electrically neutral particles and carry no charge. Of protons 17 of neutrons 37 17 20 of electrons 17 0 17.
 Source: moomoomathblog.com
Source: moomoomathblog.com
The number of subatomic particles in atoms of different elements varies. If the charge is positive there are more protons. An ion has an unequal number of protons and electrons. As summarized in Table 21 protons are positively charged neutrons are uncharged and electrons are negatively charged. I recommend watching this in x125 - 15 speed This video goes over how to know how many Protons Electrons and Neutrons are in any given element whether o.
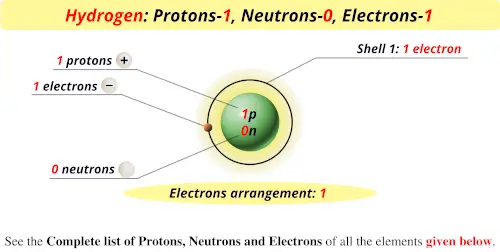 Source: periodictableguide.com
Source: periodictableguide.com
Which statement about W X Y and Z is correct. It is denoted by Z. The number of protons is equal to the number of electrons unless theres an ion superscript listed after the element. Number of protons 11. E charge on the proton and electron are exactly the same size but opposite.
 Source: britannica.com
Source: britannica.com
E charge on the proton and electron are exactly the same size but opposite. 5 Number of Neutrons. Boron-10 is composed of 5 protons 5 neutrons and 5 electrons. 23000 C 257315 K 41720 F Boiling Point. CAtomic Mass Number.
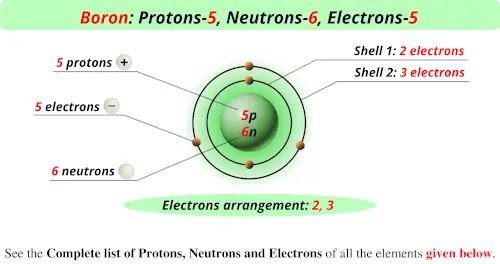 Source: periodictableguide.com
Source: periodictableguide.com
N nuclear industry boron is commonly used as a neutron absorber due to the high neutron cross-section of isotope 10B. Protons neutrons and electrons. The actual mass of an electron is very large compared to the actual mass of a proton. Electrons surround the nucleus. 21 Electrons Protons Neutrons and Atoms All matter that we are familiar with including mineral crystals is made up of atoms and all atoms are made up of three main particles.
 Source: dreamstime.com
Source: dreamstime.com
Number of protons 11. Neutrons have no charge. Answer 1 of 6. Protons are over 1800 times heavier than electrons. Of protons 17 of neutrons 37 17 20 of electrons 17 0 17.
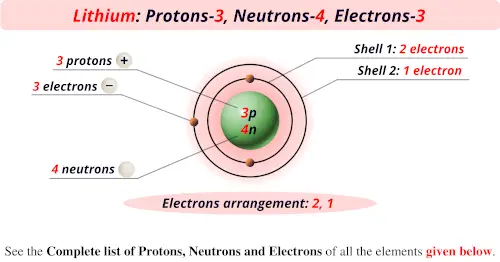 Source: periodictableguide.com
Source: periodictableguide.com
Number of neutrons mass number - atomic number 23 - 11 12. Protons have a positive charge. Atomic mass number number of protons number of neutrons. The answer to this question is in this universe but in very little quantity. All oxide anions O2- have 8 protons and 10 electrons.

The electrons are located outside the nucleus in an atom. Neutron Number and Mass Number of Boron Mass numbers of typical isotopes of Boron are 10. A neutral oxygen atom as also has 8 electrons. This means that it has gained two electrons from another element such as sodium or magnesium. Since you didnt specify which isotope Ill give the.
 Source: slideplayer.com
Source: slideplayer.com
An electron is usually. The oxide anion has a charge of 2-. The electron is a negatively charged particle found in the atoms of all the elements. Number of protons 11. N nuclear industry boron is commonly used as a neutron absorber due to the high neutron cross-section of isotope 10B.
 Source: haikudeck.com
Source: haikudeck.com
What are the Characteristics of Electron Proton and Neutron Electron Electron was discovered by JJ. The electrons are located outside the nucleus in an atom. 21 Electrons Protons Neutrons and Atoms All matter including mineral crystals is made up of atoms and all atoms are made up of three main particles. Atomic mass number number of protons number of neutrons. 25500 C 282315 K 46220 F Number of ProtonsElectrons.
 Source: szaboa-boron-project.weebly.com
Source: szaboa-boron-project.weebly.com
Cathode rays consist of small negatively charged particles called electrons. Cathode rays consist of small negatively charged particles called electrons. The protons and neutrons are found in the nucleus of the atom. Protons carry a positive electrical change while electrons are negatively charged and neutrons are neutral. An atom is normally electrically neutral and so the atomic number is also the number of electrons in a neutral atom of the element.
 Source: material-properties.org
Source: material-properties.org
This means that it has gained two electrons from another element such as sodium or magnesium. The chemical symbol for Boron is B. What are the Characteristics of Electron Proton and Neutron Electron Electron was discovered by JJ. All oxide anions O2- have 8 protons and 10 electrons. Since you didnt specify which isotope Ill give the.
 Source: rsn-msk.ru
Source: rsn-msk.ru
Rare Earth Elements Basic Information Atomic Structure Isotopes Related Links Citing This Page. 21 Electrons Protons Neutrons and Atoms All matter that we are familiar with including mineral crystals is made up of atoms and all atoms are made up of three main particles. Neutrons are electrically neutral particles and carry no charge. The 1 charge of one. Neutron Number and Mass Number of Boron Mass numbers of typical isotopes of Boron are 10.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title b element protons neutrons electrons by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






